- Company
- The dismissal of 18 employees of Zuellig Pharma is unfair
- by Jan 1, 2023 10:43pm
- According to the pharmaceutical industry on the 29th, the 11th Civil Affairs Department (chief judge Park Tae-il) of the Seoul Western District Court ruled in favor of some of the plaintiffs in a lawsuit filed by 18 fired Zuellig Pharma SSK against the company on the 22nd. According to the Democratic Pharmaceutical Union, the first trial cour
- Company
- Pharmaceutical shares capitalization of ₩56 trillion
- by Chon, Seung-Hyun Jan 1, 2023 10:42pm
- This year, pharmaceutical bio companies' stock prices were sluggish. It has never recovered from its peak on the first day of the stock market opening. The market capitalization of major companies fell by more than 56 trillion won. More than two out of ten have seen their market capitalization shrink by less than half in a year. According to the
- Company
- Korea develops 2 new drugs and 2 biosimilars in 2022
- by Chon, Seung-Hyun Dec 29, 2022 06:04am
- In Korea, domestic pharmaceutical companies succeeded in developing 2 new drugs this year. The new drugs developed by SK Bioscience and Daewoong Pharmaceuticals reached the commercialization stage. This is the most amount of biosimilars developed in Korea in 7 years since 2015. ◆SK Bioscience receives approval for the first new homegrown
- Company
- EUA for Zochova was not requested
- by Kim, Jin-Gu Dec 29, 2022 06:04am
- The government also keeps the door open to "monitoring overseas situations"...U.S. and Europe are under review. Ildong Pharmaceutical is expected to shift its strategy of introducing oral COVID-19 treatment Zochova (s-217622) in Korea from the EUA to conditional permission. On the 28th, the Central Disease Control Headquarters announced th
- Company
- Daewoong and Neurorive will codevelop new antidepressant
- by Lee, Seok-Jun Dec 29, 2022 06:03am
- On the 28th, Daewoong Pharmaceutical announced it had signed an agreement with Neurorive to conduct joint research and development for a new drug candidate for depression. Under the agreement, the two companies will be jointly developing ‘NR-0601,’ a multi-target, non-narcotic oral depression treatment. Neurorive is a bio venture com
- Company
- LegoChem Bio may receive milestone payments next yr
- by Dec 28, 2022 05:49am
- LegoChem Bioscience’s drug candidates that have been licensed out are making their way into clinical trials. The company succeeded in making licensing deals for 10 antibody-drug conjugate (ADC) pipelines and is expected to start receiving milestone payments in earnest in line with their development progress. ◆Company may earn up to
- Company
- More companies are overcoming patents for Otezla
- by Kim, Jin-Gu Dec 28, 2022 05:48am
- Pharmaceutical bio companies seeking to release generics for Otezla, a psoriasis treatment that has not been released in Korea, are expanding. In addition to the existing Daewoong Pharmaceutical and Dong-A ST, DongKoo Bio, Huons, and Chong Kun Dang succeeded in overcoming two out of three related patents. If they overcome the remaining usage
- Company
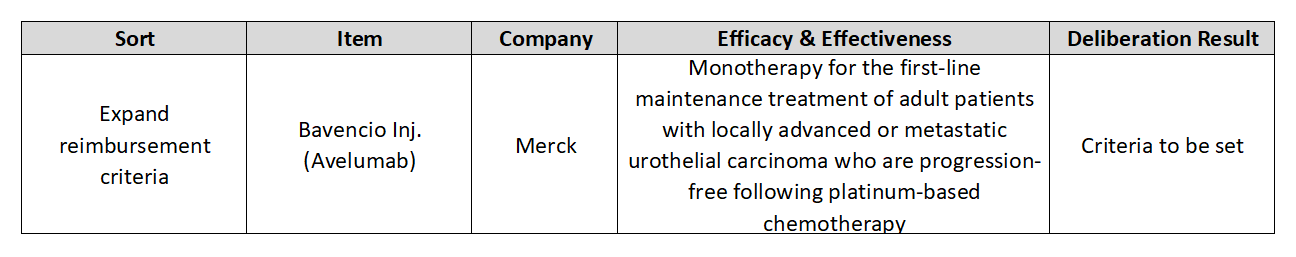
- Why no news is being heard on reimb extension for Bavencio
- by Dec 28, 2022 05:48am
- Merck and Pfizer’s cancer immunotherapy ‘Bavencio (avelumab)’ is having trouble expanding its reimbursement in Korea. Although the agenda has passed deliberations by the Cancer Disease Deliberation Committee (CDDC), the follow-up process was sluggish and no progress has been made for 8 months. According to the pharmaceutical industry on th
- Company
- Phase 1/2 of CJ Bio's Microbiome has been applied
- by Dec 28, 2022 05:48am
- CJ Bioscience announced on the 26th that it has submitted an IND of phase 1/2 of the Microbiome immuno-cancer drug "CJRB-101" to the U.S. Food and Drug Administration (FDA). CJRB-101 is a new immuno-cancer drug candidate secured by CJ Bioscience. It was developed through various immunological reviews based on the strain library that CJ has bu
- Company
- Kisqali Extends PFS by 1 yr in poor prognosis breast cancer
- by Dec 28, 2022 05:48am
- Novartis Korea announced on the 26th that the progressive and metastatic breast cancer treatment Kisqali has extended the progressive survival period by about one year compared to the first treatment of hormone receptor-positive (HR+)/human epithelial cell growth factor 2 negative (HER2-) breast cancer patients. The results of this study were