- Company
- Celltrion Stops Developing Inhalation-type COVID Antibody Tx
- by Kim, Jin-Gu Jun 29, 2022 05:53am
- Celltrion announced on the 28th that it will suspend phase 3 clinical trials of inhalation-type antibody treatments that were being developed as COVID-19 treatments. Celltrion explained the reason for the clinical suspension that the business feasibility of the COVID-19 treatment will be insignificant due to the difficult global clinical envi
- Company
- Patent challenge for gastritis drug Rebamipide expanded
- by Kim, Jin-Gu Jun 29, 2022 05:53am
- 30 generic companies participated in the patent dispute for Yuhan's acute and chronic gastritis treatment "Recomid SR." According to the pharmaceutical industry on the 24th, 20 pharmaceutical companies, including Kyung Dong, recently requested a passive judgment on the scope of rights to Recomid SR's patent. Prior to them, 13 pharmaceutical
- Company
- Kwangdong will sell GSK’s allergic rhinitis treatment
- by Chon, Seung-Hyun Jun 28, 2022 06:09am
- Kwangdong Pharmaceutical announced on the 27th that it has signed a sales partnership agreement with GSK for its allergic rhinitis treatment, ‘Avamys nasal spray.’ Avamys, which was granted marketing authorization in Korea in 2009 is a steroid nasal spray indicated for the treatment of seasonal and perennial (year-round) allergic rhinit
- Company
- Oral SMA drug Evrysdi may be prescribed at general hospitals
- by Eo, Yun-Ho Jun 27, 2022 05:58am
- The oral spinal muscular atrophy (SMA) treatment ‘Evrysdi’ may be prescribed at general hospitals. According to industry sources, Roche Korea’s SMA treatment Evrysdi (risdiplam) passed the drug committee (DC) reviews of several medical institutions including Seoul National University Hospital. Evrysdi was first approved in Korea in
- Company
- This time it’s acyclovir… impurity issue rises again
- by Chon, Seung-Hyun Jun 27, 2022 05:57am
- The drug impurity risk issue has spread to the antiviral acyclovir. According to the impurity issue that arose abroad, the health authorities have started making safety measures on pharmaceutical companies. According to industry sources on the 23rd, the Ministry of Food and Drug Safety has recently ordered pharmaceutical companies to con
- Company
- Belvarafenib confirmed its safety & effectiveness
- by Chon, Seung-Hyun Jun 27, 2022 05:57am
- A study showed that the new anticancer drug Belvarafenib, which Hanmi Pharmaceutical exported technology to Genentech, confirmed its safety and effectiveness overseas. According to Hanmi Pharmaceutical on the 22nd, Roche revealed the progress of clinical research on "Belvarafenib," which is being developed by its subsidiary Genentech, at a co
- Company
- The flu is going around in the southern hemisphere
- by Whang, byung-woo Jun 27, 2022 05:57am
- Concerns are growing that the influenza epidemic may begin as the COVID-19 pandemic turns into an endemic and social distancing such as lifting outdoor masks is eased. As the flu is showing signs of a full-fledged epidemic in Australia, one of the southern hemisphere countries that uses the flu as an indicator of the flu epidemic in the se
- Company
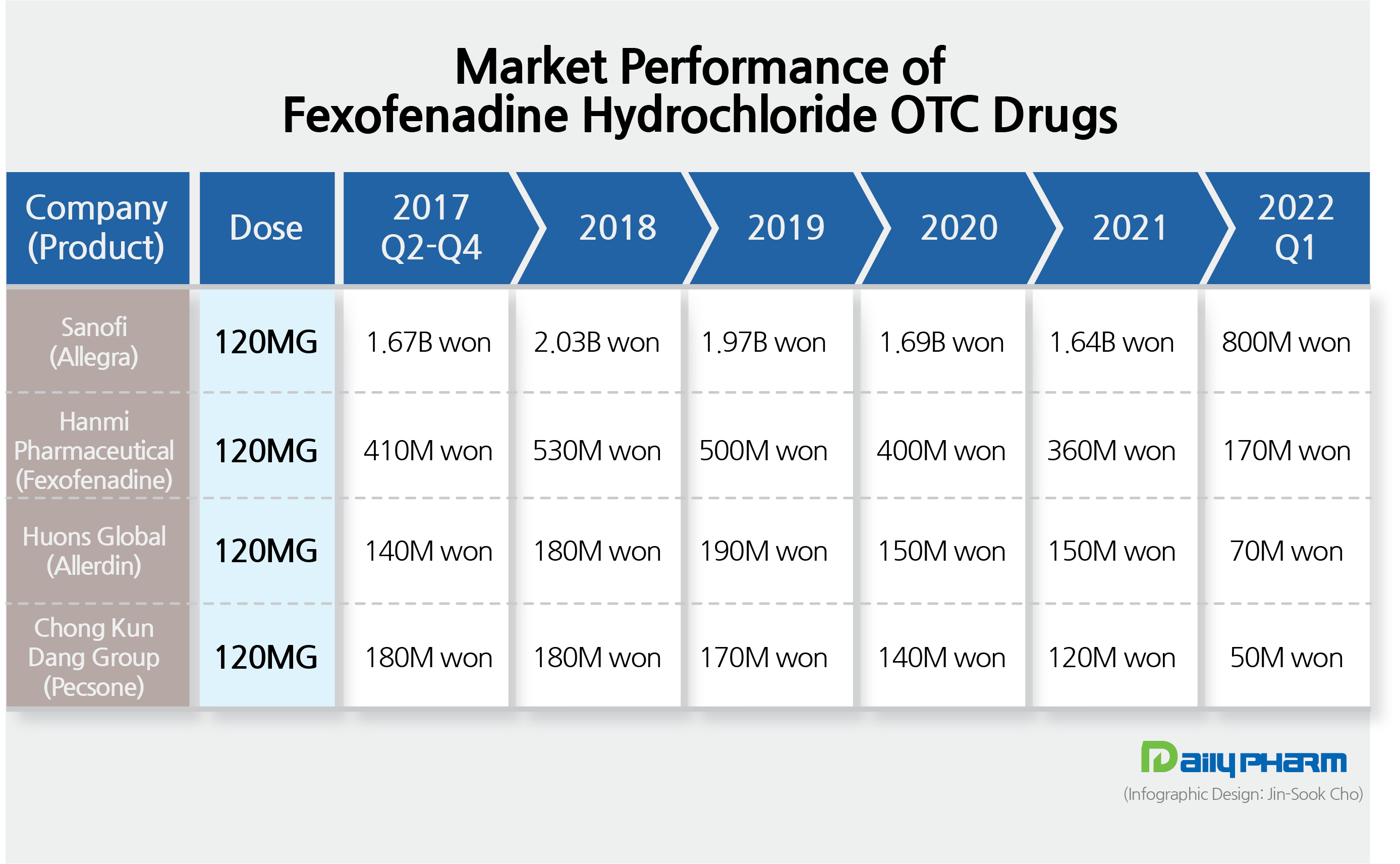
- Sanofi’s Allegra dominates OTC·ETC drug market
- by Nho, Byung Chul Jun 24, 2022 05:46am
- The market for OTCs and ETCs of the antihistamine ingredient fexofenadine hydrochloride has been showing stagnant performance with its sales making a rectangle pattern for several years now. The market is virtually monopolized by the original drug, Sanofi’s Allegra Tab., with the total market estimated to be in the &8361;8 billion range
- Company
- Yuhan’s new lung cancer drug Leclaza shows OS benefit
- by Nho, Byung Chul Jun 24, 2022 05:46am
- Yuhan Corp (CEO and President Wook Je Cho) announced on the 23rd that it had confirmed the overall survival (OS) benefit of Leclaza (lasertinib) in the Phase I/II LASER201 trial (NCT03046992). Leclaza is a treatment for epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC). Leclaza is a third-g
- Company
- LG Chem’s Zemiglo+Forxiga combo Zemidapa is approved
- by Chon, Seung-Hyun Jun 23, 2022 05:50am
- LG Chem made a public announcement on the 22nd that the company had received marketing authorization for its type 2 diabetes treatment ‘Zemidapa Tab’ from the Ministry of Food and Drug Safety. The drug is a fixed-dose combination of the antidiabetic drug gemigliptin and dapagliflozin. Gemigliptin is an active ingredient of "Zemiglo," a