- Company
- New K-drugs for metabolic diseases make international debut
- by Son, Hyung Min Jun 30, 2025 06:07am
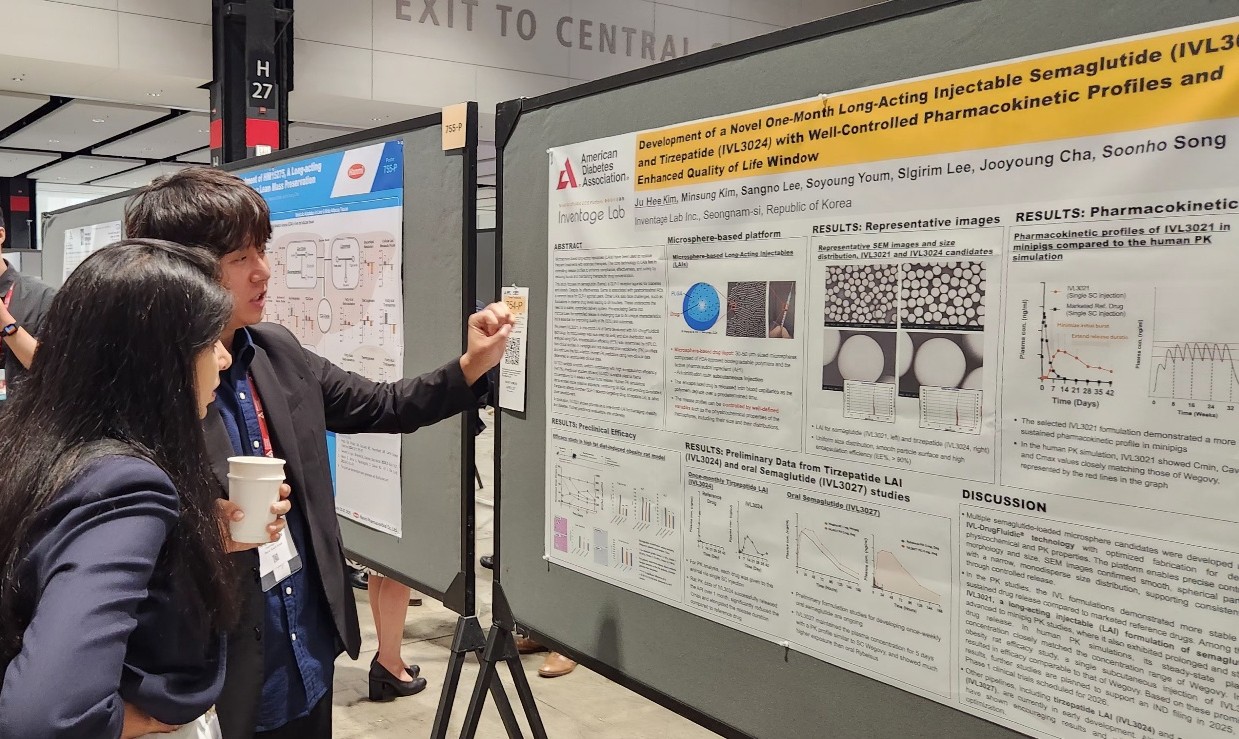
- Major Korean pharmaceutical and biotechnology companies have signaled their full-scale entry into global clinical trials, presenting new drug development results at overseas conferences. The companies presented their achievements in developing new drugs for various metabolic diseases, including obesity, type 2 diabetes, and metabolic dysfunction
- Company
- Adempas may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jun 30, 2025 06:06am
- Adempas, a new treatment for pulmonary arterial hypertension that has emerged after a long wait, is now available for prescription at general hospitals in Korea. According to industry sources, Bayer Korea's Adempas (riociguat) has been approved by the Drug Committee (DC) of tertiary hospitals in Korea, including Samsung Medical Center and
- Company
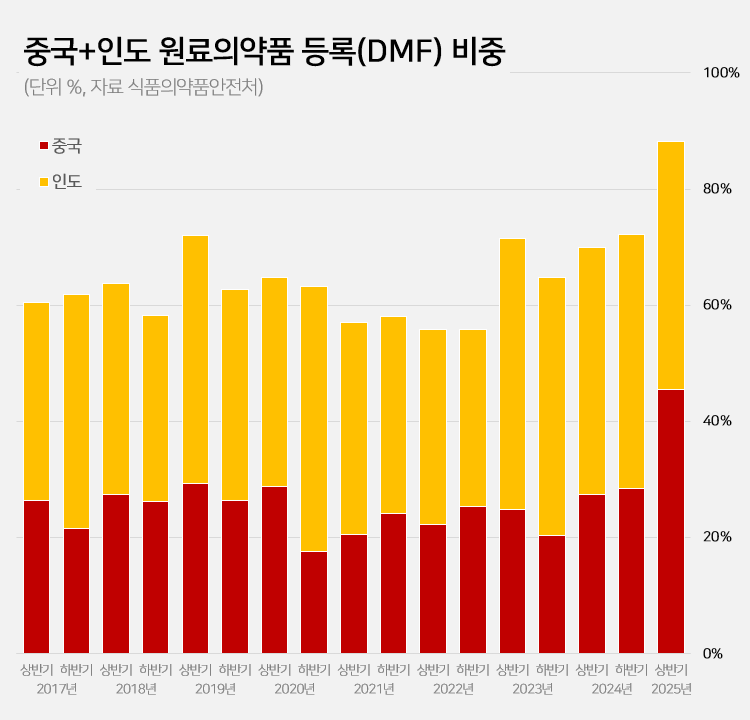
- 88% registered APIs imported from China or India
- by Kim, Jin-Gu Jun 27, 2025 06:04am
- Amid a surge in the number of drug master file registrations in the first half of this year, the share of raw materials from China and India rose to 88.2%. This is a sharp increase compared to the average of 62.1% share the two countries had during the past 5 years. This is attributed to the large number of previously delayed raw material dru
- Company
- Tevimbra adds esophageal, gastric, lung cancer indications
- by Whang, byung-woo Jun 27, 2025 06:02am
- BeiGene Korea (Name to be changed to BeOne Medicine Korea) announced that its immuno-oncology drug Tevimbra (tislelizumab) has been approved by the Ministry of Food and Drug Safety for additional indications for esophageal cancer, gastric cancer, and non-small cell lung cancer. With the additional approval, Tembriva can now be used as a fi
- Company
- KPTA ‘KOR-CHN-JPN supply cooperation to bring $12B effect'
- by Kim, Jin-Gu Jun 27, 2025 06:02am
- The Korea Pharmaceutical Traders Association (KPTA), China Chamber of Commerce for Import & Export of Medicines & Health Products (CCMPHIE), and Japan Pharmaceutical Traders Association (JPTA) announced on June 25 that they signed a memorandum of understanding (MOU) for the stabilization of the pharmaceutical supply chain at the Korea Pavi
- Company
- ‘Wegovy, a game-changer for high-risk obesity patients’
- by Whang, byung-woo Jun 26, 2025 06:08am
- Obesity is a cause of various metabolic syndromes and a major risk factor for cardiovascular disease. In fact, approximately 80% of patients hospitalized for cardiovascular disease are obese, and studies have shown that the risk of cardiovascular events in obese patients is up to twice as high as in those of normal weight. Over the past 20 ye
- Company
- The 2nd KRAS-targeted cancer drug 'Krazati' expected
- by Eo, Yun-Ho Jun 26, 2025 06:08am
- The second KRAS inhibitor is expected to be commercialized in South Korea. Bristol Myers Squibb (BMS) Korea recently submitted a marketing authorization application to the Ministry of Food and Drug Safety (MFDS) for its anti-cancer drug, Krazati (adagrasib). Krazati was also designated as an orphan drug in January. It is indicated for
- Company
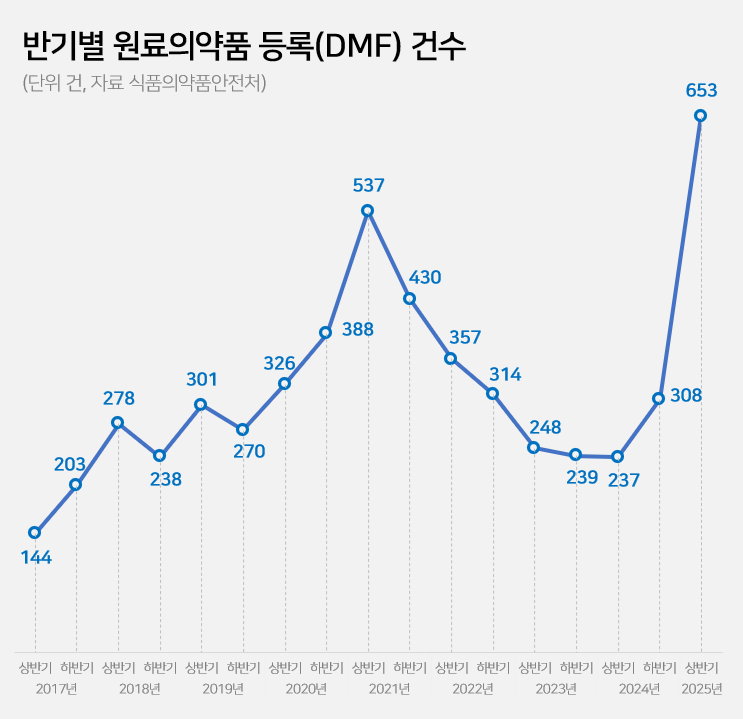
- Drug Master File for API 237→653…'easing of regulation'
- by Kim, Jin-Gu Jun 26, 2025 06:07am
- The number of Drug Master File, DMF, cases in the first half of this year surged by 2.8 times compared to the same period last year. This is the highest for a half-year period. Analysis suggests that this is due to the easing of Active Pharmaceutical Ingredient (API) registration requirements at the beginning of the year. The government had
- Company
- Vemlidy indication extended to children aged 6 and older
- by Whang, byung-woo Jun 25, 2025 06:01am
- Gilead Sciences Korea announced on the 24th that its hepatitis B treatment Vemlidy (tenofovir alafenamide, TAF) has been approved by the Ministry of Food and Drug Safety for the treatment of chronic hepatitis B in children aged 6 years and older. Vemlidy offers improved renal and bone safety compared to existing chronic hepatitis B treatm
- Company
- K-Bio successfully signs multiple technology transfer deals
- by Son, Hyung Min Jun 25, 2025 06:01am
- In the first half of this year, the Korean pharmaceutical and biotech industry has successfully achieved technology transfers in various areas, including changed formulations for immunotherapy, new obesity drug candidates, and new drug discovery platforms. Companies, such as LigaChem Biosciences and Onconic Therapeutics, have successfully receiv