- Company
- Why Vemlidy's patent dispute took 3 years to win the 1st tri
- by Kim, Jin-Gu Mar 24, 2022 05:54am
- Generics, which challenged the patent of Gilead Science's hepatitis B treatment Vemlidy (Tenofovir Alafenamide HemiFumarate), won the first trial in about three years. According to the pharmaceutical industry on the 21st, Intellectual Property recently made a "claim establishment" trial on the passive scope of the Vemliddy salt patent fil
- Company
- Samsung Bioepis makes ₩847 billion expanding overseas
- by Chon, Seung-Hyun Mar 23, 2022 05:51am
- Samsung Bioepis made a new sales record last year. With the global expansion of its biosimilars well on track, the company had made over &8361;800 billion last year. According to the Korea Financial Supervisory Service, Samsung Bioepis’s sales recorded &8361;847 billion last year, a 9.0% increase from the previous year. This is the largest
- Company
- Gov purchased ₩200 billion worth of COVID-19 vaccines
- by Chon, Seung-Hyun Mar 23, 2022 05:51am
- The COVID-19 vaccine, which incorporates technology from domestic bio companies, will be supplied for the first time in Korea. SK Bioscience announced on the 21st that it has signed a pre-purchase contract with the KDCA for the COVID-19 vaccine worth 200 billion won. The contract volume is 10 million inoculations, and SK Bioscience will seque
- Company
- Seqirus 'will introduce the first adjuvanted flu vaccine'
- by Mar 22, 2022 05:53am
- A multinational pharmaceutical company has bravely thrown its hat into the domestic influenza vaccine ring that is led by Korean companies such as GC Pharma and SK Bioscience, etc. The company, named Seqirus, is attempting to enter the Korean market equipped with the solid technology it accumulated through its sole focus on influenza vaccines.
- Company
- GSK’s immuno-oncology drug Jemperli applies for approval
- by Eo, Yun-Ho Mar 22, 2022 05:53am
- Another immuno-oncology drug is set to soon be introduced to Korea. According to industry sources, GSK Korea has applied for the approval of its PD-1 inhibitor ‘Jemperli (dostarlimab),’ and is undergoing discussions with relevant authorities. If approved, Jemperli will become the third PD-1 inhibitor to be approved in Korea after
- Company
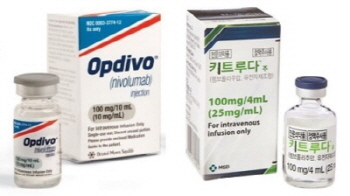
- Esophageal cancer, added to indication of Opdivo·Keytruda
- by Eo, Yun-Ho Mar 22, 2022 05:53am
- Cancer immunotherapys are advancing into the esophageal cancer area one after another. According to related industries, Opdivo (Nivolumab) and MSD's Keytruda recently were added esophageal cancer indications in Korea. Opdivo obtained final approval from the MFDS last month and Keytruda on the 7th. With this permission, Opdivo can be prescribe
- Company
- Celltrion was fined 13.9 billion won
- by Kim, Jin-Gu Mar 22, 2022 05:53am
- Financial authorities imposed a fine of 13.9 billion won on three Celltrion companies that prepared and disclosed financial statements in violation of accounting standards. The Financial Services Commission held its fifth regular meeting on the 16th and decided on this. The fines decided at the meeting are 6 billion won for Celltrion, 6.04
- Company
- Concerns over fungal infection due to COVID-19
- by Eo, Yun-Ho Mar 21, 2022 05:56am
- There is concern about the gap in fungal infections due to the re-proliferation of COVID-19. The most commonly reported fungal infections in COVID-19 patients include COVID-19 associated pulmonic aspergillosis (CAPA), COVID-19 associated mucomycosis (CAM), and Candida auris. These COVID-19-related fungal infections can lead to serious dise
- Company
- 5 companies challenge breast cancer drug Ibrance’s patent
- by Kim, Jin-Gu Mar 21, 2022 05:55am
- The number of companies challenging the patent of Pfizer’s breast cancer treatment ‘Ibrance (palbociclib)’ has increased to 5. According to the pharmaceutical industry, Boryung Pharmaceutical, Shinpoong Pharm, Daewoong Pharmaceutical, and Samyang Holdings had filed a series of claims to confirm the passive scope of rights on Ibrance’
- Company
- K-Bio has become a global production hub
- by Kim, Jin-Gu Mar 18, 2022 05:55am
- The Korean pharmaceutical bio industry has become a global coronavirus vaccine and treatment consignment production hub. With the consignment production of coronavirus vaccines and antibody treatments in charge, the company will be in charge of consignment production of oral treatments for the supply of underdeveloped countries. On the 17t