- Company
- Changes in the lung cancer treatment market are detected
- by Mar 7, 2022 05:50am
- Changes are detected in the EGFR targeted anticancer drug market, which Tagrisso dominated. Tagrisso sales stagnated for the first time due to sluggish primary treatment benefits. Leclaza, the only domestic new drug in the market, is threatening Tagrisso. According to IQVIA, a pharmaceutical research institute, on the 5th, the size of the dom
- Company
- Immuno-oncology drugs make ₩400 billion after 7 years
- by Mar 7, 2022 05:49am
- The immuno-oncology drug market exceeded &8361;400 billion in annual sales only 7 years since its debut in Korea. With Keytruda in the lead making over &8361;200 billion in sales, the latecomers Tecentriq and Imfinzi are chasing the lead with its rapid growth. According to the market research institution IQVIA on the 4th, the total imm
- Company
- Organon is focusing on expanding the women's health lineup
- by Mar 7, 2022 05:49am
- The demand for unmet diseases related to women's entire life cycle was relatively high. Organon will focus on improving treatment accessibility so that all women can enjoy healthier daily lives." At a press conference held by Organon for the first time since its launch at The Plaza Hotel in Jung-gu, Seoul on the morning of the 2nd, CEO Kim So-e
- Company
- First RSA drug ‘Erbitux’ to receive second reevaluations
- by Eo, Yun-Ho Mar 4, 2022 05:57am
- The term of risk-sharing agreements of major anticancer drugs are again nearing expiry. Starting with Merck’s ‘Erbitux (cetuximab), the first drug to be reimbursed with the RSA scheme in Korea, a series of other drugs are also awaiting reevaluations until 2023. According to industry sources, the colorectal cancer treatment Erbitux’s
- Company
- ↓↓Sales of smoking cessation txs by 75% in 4 yrs
- by Ji Yong Jun Mar 4, 2022 05:56am
- The market for Varenicline, a non-smoking supplement, has fallen to a quarter in four years. This is because the distribution of the original drug Pfizer Champix, which led the market, was suspended due to an impurity crisis in the second half of last year. According to IQVIA, a pharmaceutical research institute, sales of Varenicline last year
- Company
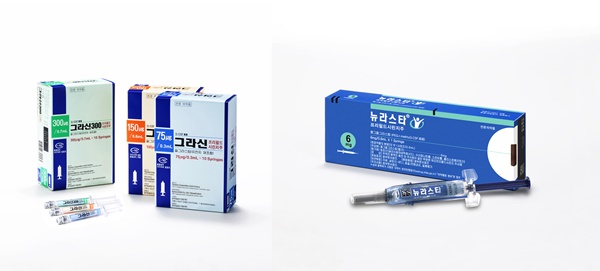
- Kyowa Kirin·Boryung to copromote sale of 2 neutropenia txs
- by Chon, Seung-Hyun Mar 4, 2022 05:55am
- Boryung Pharmaceutical and Kyowa Kirin Korea will be jointly selling 2 types of neutropenia treatments in Korea. On the 2nd, Kyowa Kirin Korea and Boryung Pharmaceutical announced that the companies signed a co-promotion agreement to jointly sell the neutropenia treatments ‘Grasin (filgrastim)’ and ‘Neulasta (pegfilgrastim)’ in Kore
- Company
- Saxenda and Qsymia tops the growing obesity market
- by Chon, Seung-Hyun Mar 3, 2022 06:01am
- The obesity treatment market has grown to break its own record for 3 consecutive years. The introduction of a new type of obesity treatment such as ‘Saxenda’ and ‘Qsymia’ had also led to market expansion.’ The two drugs have dominated the share of existing products and have occupied about half of the total market. According to market res
- Company
- BRAF I Braftovi can be prescribed at the Big 5
- by Eo, Yun-Ho Mar 3, 2022 06:01am
- Along with the progress of the insurance benefit procedure, "Braftovi," which lowers the BRAF, will be prescribed at general hospitals. According to related industries, Braftovi (Encorafenib), a treatment for colorectal cancer in Ono Pharmaceutical Industry, passed the Pharmaceutical Affairs Committee (DC) of Korea University Anam Hospita
- Company
- Entresto settles in as early-stage heart failure treatment
- by Eo, Yun-Ho Mar 3, 2022 06:01am
- The heart failure treatment ‘Entresto’ will now be covered by insurance benefits in the first line as well. According to industry sources, reimbursement for Novartis Korea’s chronic heart failure treatment ‘Entresto (sacubitril)’ will be expanded to cover patients with acute decompensated heart failure who are in a stable hemodynamic
- Company
- Omicron confirmed cases continue to occur in companies
- by Ji Yong Jun Mar 2, 2022 05:55am
- Due to the spread of COVID-19 Omicron, there are a series of in-house confirmed cases within pharmaceutical bio companies. Pharmaceutical bio companies are responding by operating the Omicron emergency system and granting additional telecommuting to close contacts. According to the Central Disease Control Headquarters on the 2nd, the numbe