- Company
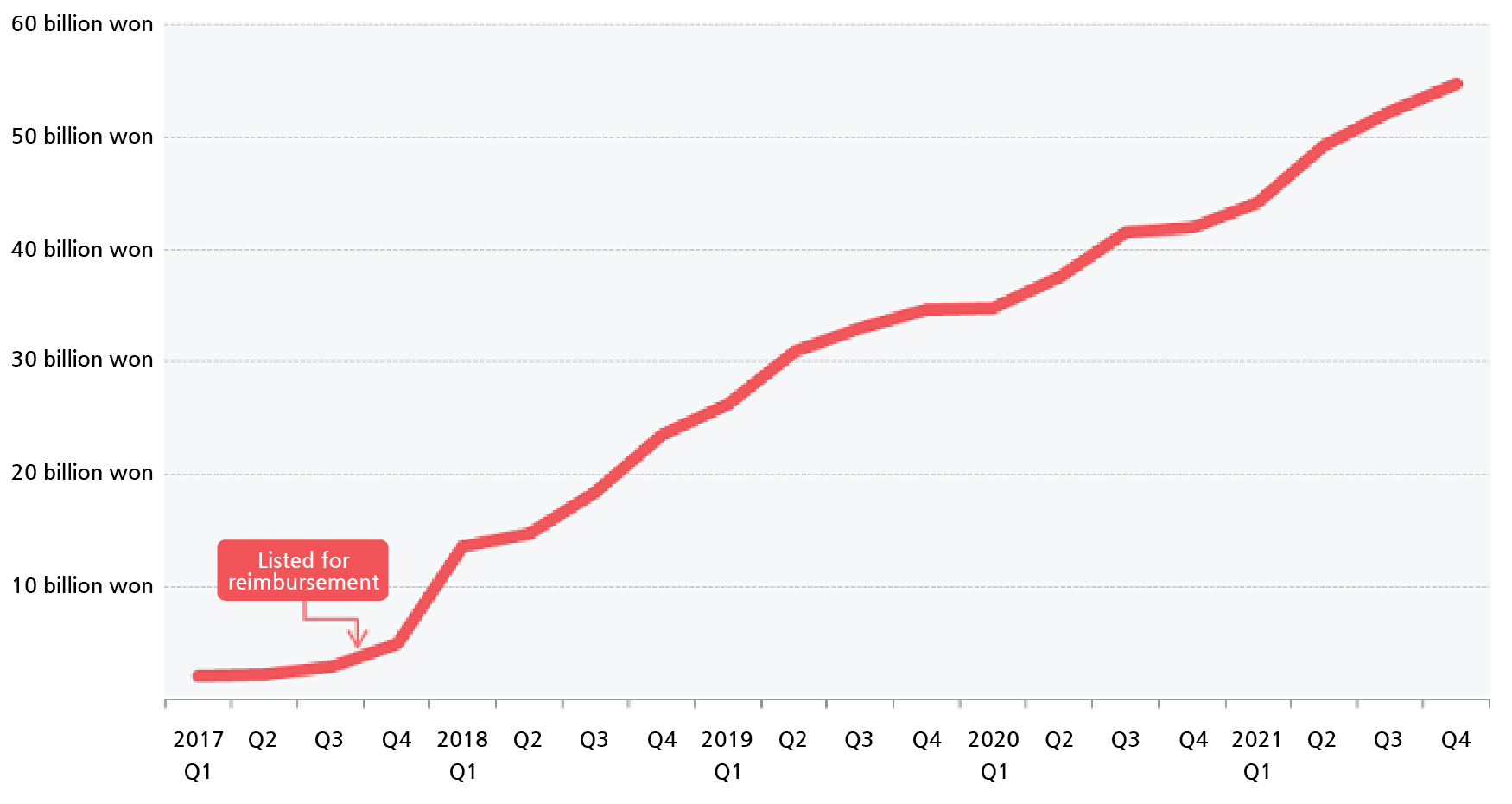
- Keytruda may make ₩300 billion with 1st-line reimb
- by Mar 2, 2022 05:55am
- The reimbursement expansion approval of Keytruda is expected to add wings to the sales growth of an already leading product in the domestic pharmaceutical market. It is expected that the company may achieve &8361;300 billion in sales with its reimbursement expansion to the first-line, which has more patients, from the &8361;200 billion sol
- Company
- Tylenol's sales amounted to ₩83.1 billion last year
- by Chon, Seung-Hyun Mar 2, 2022 05:54am
- OTC Tylenol recorded the highest sales ever. Sales more than doubled last year from the previous year due to a surge in demand for COVID-19 vaccinations. However, growth slowed in the second half of last year as demand for Tylenol was resolved. According to IQVIA, a pharmaceutical research institute, sales of the Tylenol series last year amou
- Company
- Pfizer's 3rd attempt to reimburse ATTR-CM drug ‘Vyndamax'
- by Eo, Yun-Ho Feb 28, 2022 05:55am
- The third attempt at reimbursement for Vyndamax, a new drug for transthyretin amyloid cardiomyopathy, has been made by its company. According to industry sources, Pfizer Korea had again applied for the insurance reimbursement of its new drug that is indicated for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM). This is t
- Company
- Osteoporosis should be taken care of for the rest of life
- by Feb 28, 2022 05:55am
- The Korean Society for Bone and Mineral Research has begun to improve awareness of osteoporosis treatment. This is to enable continuous treatment by recognizing the seriousness of diseases that can lead to death from fractures and improving standards. Osteoporosis is a disease in which holes are formed in bones, and when bone strength weak
- Company
- Moderna and BeiGene joined KRPIA
- by Eo, Yun-Ho Feb 25, 2022 05:57am
- According to related industries, U.S.-Moderna Korea and Chinese-BeiGene Korea recently joined as KRPIA members. As a result, the number of KRPIA member companies has increased to 46. Both Moderna and BeiGene are multinational pharmaceutical companies that established a Korean subsidiary last year, and promotional activities are expected to
- Company
- Shionogi COVID tx developed by Ildong has been approved
- by Kim, Jin-Gu Feb 25, 2022 05:56am
- Ildong Pharmaceutical announced on the 23rd that it has been approved by the MFDS to change the clinical trial plan of Shionogi's oral COVID-19 treatment candidate "S-217622" under development in Korea. &8203; Earlier, Ildong Pharmaceutical applied to change its clinical plan in the direction of confirming the results of each phase by modi
- Company
- Yuhan’s ‘Leclaza’ makes ₩4.1 billion in 6 months
- by Chon, Seung-Hyun Feb 24, 2022 05:58am
- Yuhan Corporation's new drug ‘Leclaza’ has made &8361;4.1 billion in its first year of release. In only 6 months since its sales began in earnest with reimbursement listing in July, Leclaza rose to the top among homegrown anticancer drugs, raising expectations on its potential for commercial success. According to the pharmaceutical rese
- Company
- It was improved by changing the targeted therapy formulation
- by Eo, Yun-Ho Feb 23, 2022 05:50am
- According to related industries, Pfizer Korea's breast cancer treatment Ibrance(Palbociclib) has obtained an item license in a type of tablet not a capsule, six years after its launch in Korea. Also, Takeda is also preparing to introduce a tablet formulation of "Zejula (Niraparib toosylate monohydrate)," a treatment for ovarian cancer.
- Company
- Lilly-RosVivo signed a contract to transfer diabetes txs
- by Eo, Yun-Ho Feb 23, 2022 05:49am
- Nexturn Bio announced on the 22nd that its U.S. subsidiary RosVivo Therapheutics has signed a "MTA (Material Transfer Agreement)" with Eli Lilly for commercial development of a new drug candidate for diabetes treatment, "RSVI-301. MTA is a contract concluded by the counterparty to verify the efficacy and research results of the drug through e
- Company
- Endless evolution of cancer immunotherapy
- by Eo, Yun-Ho Feb 22, 2022 05:54am
- According to related industries, news of approval for the expansion of domestic indications of cancer immunotherapy Opdivo (Nivolumab) and Keytruda (Pembrolizumab) in PD-1 inhibition mechanisms continues. Although it is the same mechanism, it is competing by securing different indications. In the case of Opdivo, two postoperative adjuvant the