- Company
- Galderma challenges the atopic dermatitis market in KOR
- by Whang, byung-woo May 29, 2025 05:52am
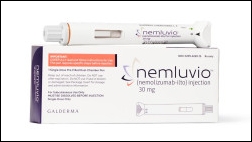
- Galderma is challenging the Korean market for atopic dermatitis treatment with its first biologic, 'Nemluvio (nemolizumab).' Given the market's already competitive nature, Galderma is likely to secure a market presence with the drug's mechanistic differentiation, convenient administration, and expanded indication. Galderma Korea submitt
- Company
- BeiGene announces name change to 'BeOne Medicines'
- by Whang, byung-woo May 29, 2025 05:51am
- BeiGene announced on May 28 that it has relaunched as BeOne Medicines Ltd., a company registered in Switzerland, along with its new name. This name change marks a significant milestone for the company and is part of a broader effort to strengthen its identity within the global biopharmaceutical industry. BeiGene Korea, the domestic entity,
- Company
- Global expansion in sight for organoid leader CellArtgen
- by Whang, byung-woo May 28, 2025 05:58am
- Organoids, miniature organs composed of cells, are emerging as a technology to replace animal testing in the pharmaceutical and biotechnology industry.&8232; With the field of organoids gaining attention, the activities of CellArtgen, which was founded by Cho Seung-woo, a leading expert in the field and professor at Yonsei University's Depar
- Company
- KDDF successfully holds 2025 KDDF Global Biotech Showcase
- by Whang, byung-woo May 28, 2025 05:55am
- [The Korea Drug Development Fund (KDDF, CEO Yeong-Min Park) announced on the 27th that it will hold the '2025 KDDF Global Biotech Showcase' to attract overseas investment for excellent new drug candidates developed in Korea. The showcase, which will be held over two days from May 27 to 28 at the Fairmont Ambassador Seoul Hotel, will focus
- Company
- Novartis Korea holds Cosentyx symposium at KCR 2025
- by Whang, byung-woo May 28, 2025 05:54am
- Novartis Korea announced on the 27th that it held a luncheon symposium at the 45th Korean Congress of Radiology Annual Scientific Meeting (KCR 2025) to highlight the clinical value of Cosentyx (secukinumab) as a treatment for ankylosing spondylitis. The luncheon symposium was moderated by Professor Yong-Beom Park of the Department of Rheu
- Company
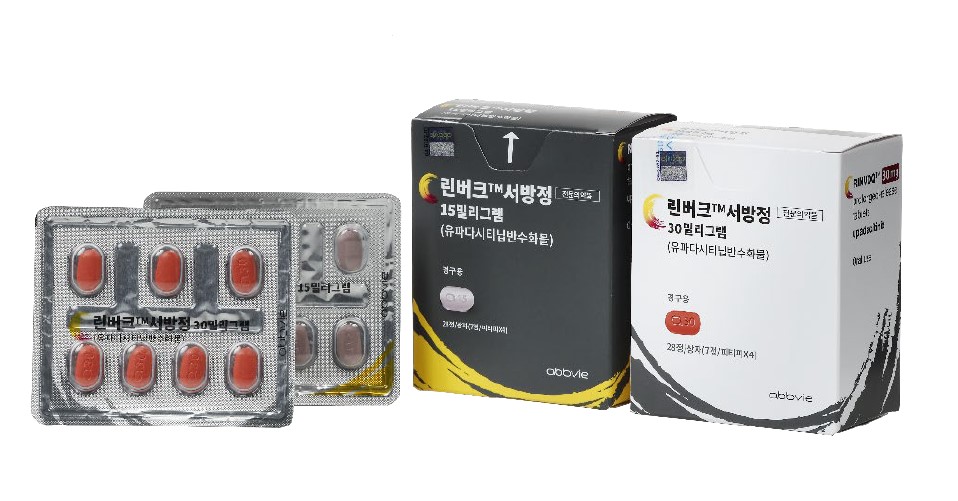
- Will JAK inhibitors for inflammation expand mkt presence?
- by Moon, sung-ho May 28, 2025 05:54am
- It has been confirmed that Janus kinase (JAK) inhibitors are more effective at rapidly and powerfully controlling inflammatory responses in atopic dermatitis compared to biologics. Now that switching therapies between different drug classes is allowed, this finding is expected to serve as a key basis for drug selection in clinical practice.
- Company
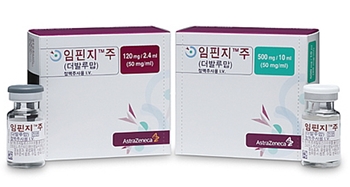
- Will Imfinzi finally be reimbursed for biliary tract cancer?
- by Eo, Yun-Ho May 27, 2025 06:18am
- With the advent of an era in which a single drug is used for multiple indications, awareness is growing on the need to address the issue of non-reimbursed indications. In particular, in order to improve Korea’s rigid reimbursement evaluation system, which is regarded as the main cause of reimbursement delays, not only using the flexible
- Company
- K-Bios head to ASCO…anticancer drugs to AI predictions
- by Kim, Jin-Gu May 27, 2025 06:18am
- Korean pharmaceutical and biotech companies set out to participate in the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, which is just 5 days away. At ASCO 2025, LG Chem's U.S. subsidiary Aveo Oncology, along with Tium Bio, Onconic Therapeutics, and ImmuneOncia, will each present clinical trial results about their anti
- Company
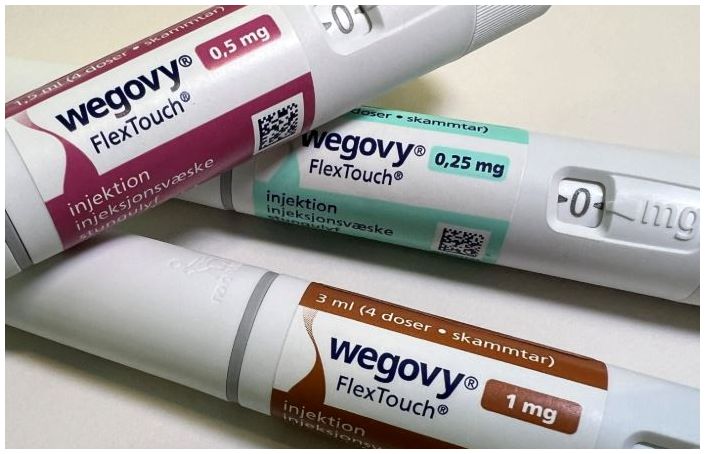
- 'Wegovy' dominating the South Korean obesity drug market
- by Chon, Seung-Hyun May 27, 2025 06:17am
- Novo Nordisk's Wegovy has dominated the South Korean obesity treatment market, establishing a monopolistic competition with over 70% market share. In just six months since its launch in Korea, Wegovy generated a sensation, surpassing KRW 100 billion in cumulative sales. Wegovy's success has expanded the obesity drug market to its largest size ev
- Company
- Re-evaluation possibility of 'Bylvay' gathers attention
- by Eo, Yun-Ho May 26, 2025 05:57am
- Attention has been drawn to when 'Bylvay Cap,' the first medicine chosen for the 'Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations,' will be re-evaluated. Bylvay (odevixibat), Ipsen Korea's treatment option for progressive familial intrahepatic cholestasis (PFIC) in patients a