- Company
- Janssen MA Director Lim Kyunghwa promoted to Janssen APAC
- by Eo, Yun-Ho Sep 24, 2020 11:59am
- Market Access (MA) Director Lim Kyunghwa at Janssen Korea is to be promoted to Janssen Asia Pacific. The pharmaceutical industry sources reported, Director Lim would be appointed as a new MA Director at Janssen Asia Pacific from coming October. Temporarily, Lim would be remotely working from South Korea amid COVID-19, but she would be wo
- Company
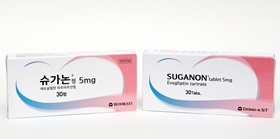
- Suganon by Dong-A ST, approved for Phase II/III in the US
- by An, Kyung-Jin Sep 24, 2020 06:19am
- Dong-A ST announced on the 22nd that the diabetes treatment 'Suganon' (Evogliptin) has been approved by the US Food and Drug Administration (FDA) for Phase IIb/IIIa clinical trial plan (IND) for aortic heart valve calcification. Suganon is a diabetes treatment based on DPP-4 inhibitor developed by Dong-A ST. In order to use Suganon other
- Company
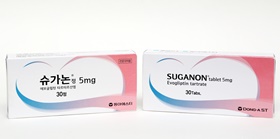
- Suganon, a new domestic drug that is evolving
- by An, Kyung-Jin Sep 23, 2020 08:06pm
- Suganon (Evogliptin), a diabetes treatment developed by Dong-A ST's proprietary technology, has stepped up its competitiveness. Despite the return of rights, efforts are being made to improve the value of new drugs while seeking new indications. The growth of domestic sales has gained momentum, and it is advancing into overseas markets by co
- Company
- Samsung signed manufacturing collaboration with AZ
- by Eo, Yun-Ho Sep 22, 2020 05:47pm
- AstraZeneca and Samsung Biologics announced the signing of a long-term supply agreement. Under this agreement, valued at approximately $330.8 million, Samsung Biologics will provide large-scale commercial manufacturing for drug substance and drug product of AstraZeneca biologics therapeutics. The contract value could grow to $545.6 millio
- Company
- Cyramza+Tarceva shoots for first-line in EGFR NSCLC patients
- by Eo, Yun-Ho Sep 22, 2020 06:27am
- A combined targeted therapy is seeking for healthcare reimbursement as a first-line treatment in patients with non-small cell lung cancer (NSCLC). The pharmaceutical industry source reported Lilly Korea has recently submitted an application to expand reimbursement on a vascular endothelial growth factor (VEGF) receptor 2 antagonist Cyra
- Company
- Ho-jin Choi inaugurated as CEO of Ono Pharma Korea
- by An, Kyung-Jin Sep 22, 2020 06:26am
- Ono Pharma Korea said that it has elected Choi Ho-jin, vice president of Ono Pharma Korea, as its new chief executive officer effective on Oct. 1. Ono Pharma Korea was established in December 2013 as a Korean subsidiary of Ono Pharmaceutical Co., Ltd. in Japan. New CEO Choi has contributed significantly to the local launch and reimbursemen
- Company
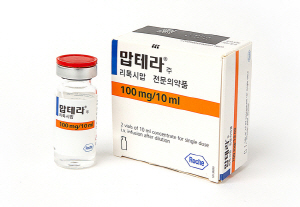
- Combination therapy of Mabthera is on the rise
- by Eo, Yun-Ho Sep 22, 2020 06:26am
- In the field of Chronic Lymphocytic Leukemia (CLL), a combination therapy of a new drug and 'Mabthera (Rituximab)' is attracting attention. According to related industries, the combination therapy of 'Imbruvica (Ibrutinib)' and Mabthera recently obtained approval in Europe after the US. The HIRA’s Pharmaceutical Benefits Advisory Committ
- Company
- Korean companies take drug patent strategy to next level
- by Kim, Jin-Gu Sep 22, 2020 06:26am
- The South Korean pharmaceutical companies are evolving their patent strategies. In the first half of the year, the companies set the historic record of registering the most number of patents. Apparently, their number of patents is on par with multinational pharmaceutical companies’. According to Ministry of Food and Drug Safety (MFDS) and K
- Company
- Sales in the outpatient prescription drug market are active
- by Chon, Seung-Hyun Sep 21, 2020 06:13am
- It has been shown that the outpatient prescription drug market has not been hit even with the recent spread of COVID-19) patients. Even though sales activities contracted due to level 2 social distancing, the total prescription drug volume recorded a growth trend last month. According to UBIST, a drug research institute on the 20th, the amoun
- Company
- Lilly's Olumiant, is clinically approved for COVID-19
- by Kim, Jin-Gu Sep 18, 2020 06:30am
- Eli Lilly's autoimmune disease treatment 'Olumiant (Baricitinib)' has begun phase III clinical trial targeting COVID-19 patients in Korea. It is the second clinical trial in Korea related to COVID-19 after Remdesivir by Gilead Science. On the 7th, the MFDS approved a phase III clinical trial plan of Baricitinib (LY3009104) in Lilly Korea f