- Company
- Venclexta will be prescribed in major hospitals soon
- by Eo, Yun-Ho Mar 12, 2020 06:07am
- Venclaxta, which is ready for insurance benefits, will be prescribed at a general hospital soon. According to the related industry, Abbvie's Chronic Lymphocytic Leukemia (CLL) treatment, Venclexta (Venetoclax), passed DC (drug committee) of 5 big major Hospitals such as Seoul National University Hospital, Seoul St. Mary's Hospital, Severan
- Company
- “No changes in safety and efficacy of montelukast”
- by Kim, Jin-Gu Mar 12, 2020 06:07am
- Medical academics argue there is “no change in safety and efficacy of montelukast,” currently controversial with safety risk. Director Kim Chang Geun of Inje Uniersity Sanggye Paik Hospital the Asthma and Allergies Center spoke on Mar. 9 at a roundtable with press. The President of Pneumonia and Respiratory Diseases Study Group at
- Company
- Household brand pain reliever Geworin price goes up
- by Jung, Hye-Jin Mar 12, 2020 06:03am
- Household brand pain reliever Geworin’s price would be raised by 8 percent to 9 percent around March or April. According to pharmacy and pharmaceutical distributor sources, Samjin Pharm has decided to adjust supply price of Geworin after contemplating on it for months. In this week, the company would officially notify the decision to wh
- Company
- Next-gen insulin medications approved for pediatric patients
- by Eo, Yun-Ho Mar 11, 2020 06:27am
- Next generation insulin medications are actively seeking for expanded indication to treat children and adolescents. Novo Nordisk’s rapid-acting insulin Fiasp FlexTouch (insulin aspart) has recently won indication to treat to children aged under two and adolescents. Fiasp is a next-generation fast-acting mealtime insulin injection that
- Company
- Celltrion, apply for European license for CT-P17
- by An, Kyung-Jin Mar 11, 2020 06:27am
- Celltrion is the first in the Humira (Adalimumab) market for its global sales of &8361;20 billion. Celltrion announced on the 9th that it has completed application for Humira biosimilar 'CT-P17' to the European Medicines Agency (EMA) on the 6th (local time). Humira, the original drug of CT-P17, is the most sold blockbuster product in t
- Company
- Drug industry's ‘Corona Blue,’ “We miss our mundane life"
- by Chon, Seung-Hyun Mar 11, 2020 06:27am
- 50 days have passed since the 2019 novel coronavirus (COVID-19) started infecting Korea. COVID-19 has consumed the whole of Korea in merely 20 days after 31st patient was confirmed with infection on last Feb. 19. As of last Sunday 4 p.m., 7,313 people were confirmed with COVID-19 in Korea. As the COVID-19 outbreak has been lingering l
- Company
- 15 domestic pharmaceuticals, focus on 'COVID-19' treatment
- by Nho, Byung Chul Mar 11, 2020 06:27am
- Domestic pharmaceutical bio companies and government agencies are concentrating on developing COVID-19 vaccines and treatments. In response to COVID-19, opinions are urged to strengthen government support and expand public-private cooperation in order to preemptively respond to similar infectious diseases. According to the KPBMA, 15 domes
- Company
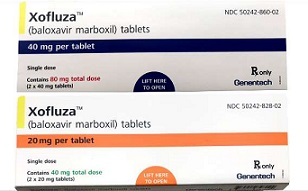
- Next-gen flu drug Xofluxa revs up for reimbursement listing
- by Eo, Yun-Ho Mar 10, 2020 06:29am
- Next-generation flu treatment Xofluza is now seeking for National Health Insurance reimbursement. Pharmaceutical industry sources reported, Roche Korea has submitted a reimbursement listing application for Xofluza (baloxavir). Finally approved after two-decade-long reign of Tamiflu (oseltamivir), Xofluza grabbed the industry’s atten
- Company
- How long do pharmaceuticals have to work from home?
- by Kim, Jin-Gu Mar 10, 2020 06:28am
- More domestic pharmaceutical companies have decided to work from home to prevent the spread of COVID-19. Following Dong-A ST and GC Pharm, Chong Kun Dang joined the ranks of all employees from home. Chong Kun Dang said five days ago that it will start working from home for all employees. it will be up to the 13th. Chong Kun Dang and its af
- Company
- Verzenio pricing negotiation to overlap with Ibrance
- by Eo, Yun-Ho Mar 9, 2020 08:00am
- After passing Ibrance, Health Insurance Review and Assessment Service (HIRA) continuously passed Verzenio. The newly passed drug would likely to overlap its pricing negotiation with Ibrance. Korean pharmaceutical industry source reported Lilly’s cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor Verzenio was given a green light for Nati