- Company
- Anti-obesity drug market stirred by lorcaserin safety issue
- by Jung, Hye-Jin Jan 22, 2020 06:27am
- Anti-obesity drug companies are on high alert as a safety issue has surfaced regarding lorcaserin, an active ingredient used for in anti-obesity medication.. Lorcaserin competitors are also getting busy anticipating doctors and pharmacists to avoid Ildong Pharmaceutical’s Belviq (locarserin), which was considered a safe anti-obesity drug
- Company
- AstraZeneca retries expanding Tagrisso's reimbursement
- by Eo, Yun-Ho Jan 21, 2020 06:25am
- Target therapy Tagrisso is trying to resume the reimbursement review procedure for its indication as a first-line lung cancer treatment. According to pharmaceutical industry source, AstraZeneca Korea has submitted a reimbursement expansion application at the end of last year for the first-line indication of its epidermal growth factor re
- Company
- Prostate cancer: New battlefield for global companies
- by Eo, Yun-Ho Jan 21, 2020 06:24am
- Prostate cancer market is predicted to emerge as another tight battleground for multinational pharmaceutical companies. Pharmaceutical industry sources reported on Jan. 20, two new drugs are to compete head-to-head with the world’s first oral option of androgen receptor (AR) inhibitor Xtandi (enzalutamide) supplied by Astellas Pharma Ko
- Company
- The 38th JP Morgan Healthcare Conference ended
- by Kim, Jin-Gu Jan 21, 2020 06:24am
- The 38th JP Morgan Healthcare Conference ended. This year, more than 30 Korean pharmaceutical bio companies visited San Francisco, USA. Seven companies, including Samsung BioLogics, Celltrion, Hanmi Pharm, and LG Chem, made public announcements. Other pharmaceutical companies also had a competition behind the curtains There are thre
- Company
- “Novartis Korea is ‘guilty,’ but directors ‘not guilty'"
- by Eo, Yun-Ho Jan 21, 2020 06:24am
- The court ruled ‘guilty’ for the company and ‘not guilty’ for the individual. The three-year-long legal dispute over Novartis Korea’s allegation of providing illegal rebate was concluded as the court found only some of them guilty. A final decision was made at 10 a.m. on Jan. 17 regarding Novartis Korea allegedly paying billions of
- Company
- The Rx rate of Lipitor and Plavix is high
- by Chon, Seung-Hyun Jan 20, 2020 06:27am
- Last year, the strength of patent expiration drugs stood out in the top domestic outpatient drug market. Lipitor, a hyperlipidemia treatment drug, continued to rise and remained No.1 in the prescription market for two consecutive years. The antithrombotic drug Plavix has grown by about 50% in five years. 'Twynsta', 'Crestor', and 'Aricept' also
- Company
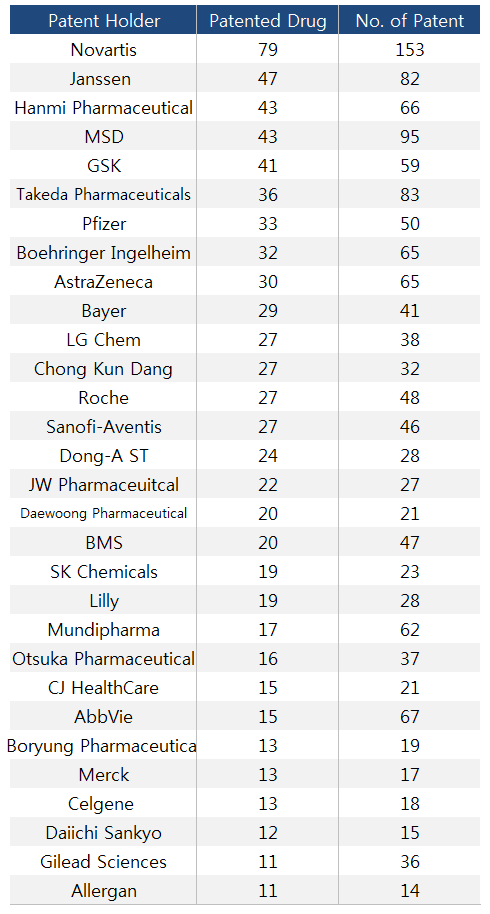
- Novartis holds the most number of drug patents in Korea
- by Kim, Jin-Gu Jan 20, 2020 06:26am
- Sources confirm a pharmaceutical company with the highest number of pharmaceutical patent in Korea is Novartis Korea. The company owns the rights of 153 patents related to total of 29 drug items. While multinational drug companies are mostly dominating the top ten list of patent holders, Hanmi Pharmaceutical with incrementally modified drug
- Company
- Takeda's Kynteles indicated for first-line treatment
- by Eo, Yun-Ho Jan 20, 2020 06:26am
- Kynteles would be rubbing shoulders with tumor necrosis factor-alpha (TNFα)-antagonist in treating ulcerative colitis and Crohn’s disease. Takeda Pharmaceuticals Korea (CEO Moon Hee-seok) official stated on Jan. 15, ulcerative colitis and Crohn’s disease treatment Kynteles (vedolizumab) won Korean Ministry of Food and Drug Safety’s (
- Company
- Hanmi to revive investigation drug returned from Janssen
- by An, Kyung-Jin Jan 20, 2020 06:26am
- Hanmi Pharmaceutical is reworking on GLP-1 dual receptor agonist Janssen has returned the rights for. Instead of targeting obesity and diabetes at once, the investigational drug shifted the focus of development as a new once-weekly dual mechanism of action to treat patients with obesity. The company aims to develop the world’s first once-w
- Company
- Samsung BioLogics opens new CDO lab in San Francisco
- by Lee, Seok-Jun Jan 20, 2020 06:25am
- Samsung BioLogics will establish a CDO R&D Lab in San Francisco in the first half of this year. The company is the first US corporation. Samsung BioLogics plans to further expand to other regions of the US and Europe. Samsung Biologics President Tae-han Kim and Vice President John Lim announced the plan at the JP Morgan Biohealth Conference i