- Company
- Beijing Hanmi Pharm posts two-fold sales·three-fold profit
- by Kim, Jin-Gu Mar 26, 2025 06:00am
- Beijing Hanmi Pharmaceutical, a local subsidiary of Hanmi Pharm in China, posted sales that approximately doubled over the past four years. The net income for the year increased more than threefold. Despite staggering performance last year, the company anticipated to be a temporary one. According to the Financial Supervisory Service on March
- Company
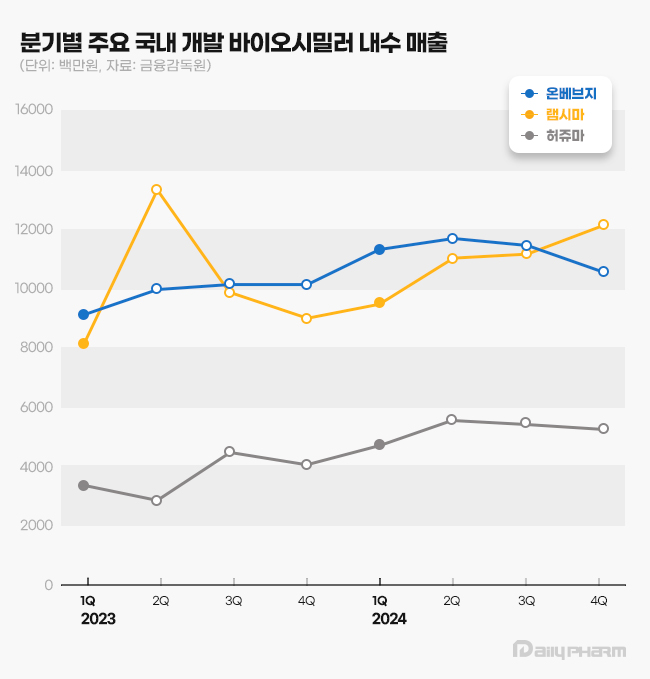
- 'Onbevezy' holds the No.1 spot for the domestic mkt
- by Chon, Seung-Hyun Mar 26, 2025 06:00am
- Samsung Bioepis' anticancer agent Onbevezy climbed to hold the No.1 spot in domestic sales as the first domestically developed biosimilar. It has surpassed Remsima for the first time. The quarterly sales of Onbevezy and Remsima exceeded KRW 10 billion, each competing to hold the No.1 spot. According to the Financial Supervisory Service on Mar
- Company
- Will topical JAK inhibitors be launched?
- by Son, Hyung Min Mar 25, 2025 05:57am
- Topical formulation of Janus kinase (JAK) inhibitors with the substantial advantage of administration convenience is entering the market for atopic dermatitis. Unlike oral formulations, a topical drug formulation can be directly applied to the skin. Thus, it has significantly improved treatment convenience. To date, Incyte's Opzelura is the o
- Company
- Adempas’s nears reimb nearly 10 years after approval
- by Eo, Yun-Ho Mar 25, 2025 05:54am
- The reimbursement of the pulmonary arterial hypertension drug 'Adempas' is near in Korea, 10 years after its approval. The National Health Insurance Service is currently negotiating with Bayer Korea for its Adempas (riociguat). However, the negotiations are expected to be concluded and the drug listed soon. The negotiations for Adempas
- Company
- HLB shares plummet upon 2nd FDA rejection of rivoceranib
- by Cha, Jihyun Mar 25, 2025 05:54am
- The shares of HLB Group affiliates plummeted as HLB's new drug for liver cancer failed to enter the US market again. The total market capitalization of HLB Group stocks evaporated by over KRW 3 trillion in a single day. However, this did not cause a simultaneous drop in domestic bio stocks. HLB Group's 10 listed affiliates evaporate by KR
- Company
- The first RSV vaccine 'Arexvy' to launch in May in Korea
- by Whang, byung-woo Mar 25, 2025 05:54am
- As the launching date of Arexvy, known as the first respiratory syncytial virus vaccine, has been announced, the company aims to challenge a market share. According to industry sources on March 22, GSK Korea has confirmed the launching date of the RSV-LRTD vaccine, Arexvy, as May. Arexvy received approval from the Ministry of Food and D
- Company
- 'Xeljanz' reimbursed for juvenile idiopathic arthritis
- by Eo, Yun-Ho Mar 24, 2025 05:52am
- 'Xeljanz' has become the first JAK inhibitor to be reimbursed for the treatment of juvenile idiopathic arthritis. The Ministry of Health and Welfare (MOHW) has recently announced on the administrative notification board regarding the 'The Criteria and Scope of National Health Insurance (Pharmaceuticals)' that the reimbursement criteria fo
- Company
- RSV vaccine Beyfortus lands in Big 5 Hospitals in Korea
- by Eo, Yun-Ho Mar 24, 2025 05:52am
- The respiratory syncytial virus (RSV) preventive antibody injection ‘Beyfortus’ has landed in the Big 5 tertiary hospitals in Korea. According to industry sources, Sanofi Korea's Beyfortus (nirsevimab) has passed the drug committees (DCs) of the Big 5 tertiary hospitals in Korea, including Samsung Medical Center, Seoul National Universi
- Company
- Bispecific multiple myeloma drug 'Talvey' can be prescribed
- by Eo, Yun-Ho Mar 21, 2025 06:00am
- New bispecific multiple myeloma drug 'Talvey' can now be prescribed at general hospitals. According to industry sources, Janssen Korea's Talvey (talquetamab) has passed the drug committees (DC) of tertiary general hospitals, including Samsung Medical Center and Seoul National Univeristy Hospital, and medical institutes, including Pusan Na
- Company
- Prevnar 20 added to the National Immunization Program
- by Whang, byung-woo Mar 21, 2025 05:59am
- Pfizer is signaling full-fledged competition with the addition of its new pneumococcal vaccine, Prevnar 20, to the National Immunization Program (NIP) for children. According to industry sources on the 21st, the Korea Disease Control and Prevention Agency recently reviewed the introduction of PCV20 NIP for children during the 1st Korea Exp