- Company
- Alteogen signs 2 licenses out agreements with AZ subsidiary
- by Cha, Jihyun Mar 18, 2025 05:56am
- Alteogen has signed two licensing-out agreements with AstraZeneca’s subsidiaries. According to the Financial Supervisory Service on the 17th, Alteogen has signed 2 exclusive license agreements with MedImmune, a subsidiary of AstraZeneca's bio R&D, for its subcutaneous (SC) formulation modification platform 'ALT-B4' based on recombinant h
- Company
- Can the myelofibrosis drug Ojjara pass CDDC review in KOR?
- by Eo, Yun-Ho Mar 18, 2025 05:56am
- Industry attention is rising on whether GSK’s new drug for myelofibrosis, Ojjara, will be able to make progress in its reimbursement journey and be listed in Korea. According to industry sources, GSK's myelofibrosis treatment Ojjara (momelotinib) will be presented for review to the Health Insurance Review and Assessment Service's Cancer
- Company
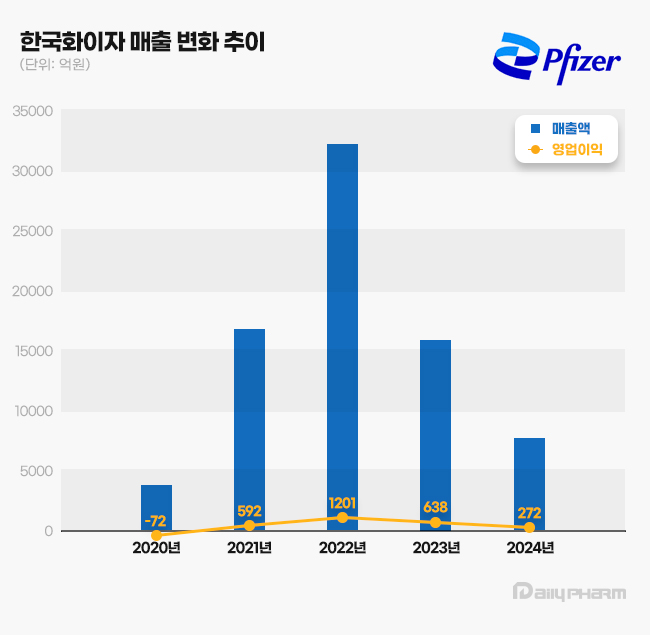
- Pfizer distributed KRW 160 billion dividend over 2 years
- by Chon, Seung-Hyun Mar 18, 2025 05:56am
- Pfizer Korea has decided on a large-scale dividend payout for the second consecutive year. While the company maintained an annual dividend policy of KRW 12.48 million, it has distributed KRW 160 billion to its parent company over the past two years. Large-scale profits during the COVID-19 pandemic enabled this high dividend. According to the
- Company
- Sanofi 'Dupixent' adds COPD indication
- by Whang, byung-woo Mar 17, 2025 06:00am
- 'Dupixent,' dominating the market for atopic dermatitis·asthma drugs, has expanded treatment areas to chronic obstructive pulmonary disease (COPD), thus gaining attention. Since Dupixent was approved for expanded reimbursement last year, the latest news on COPD indication will likely improve its prescription competitiveness. According
- Company
- K-Bios busy developing new CAR-NK cell therapies
- by Son, Hyung Min Mar 17, 2025 06:00am
- The domestic pharmaceutical industry is initiating clinical trials for cell therapies and confirming new possibilities. GC Cell, Isu Abxis, Vaxcell Bio Therapeutics, GI Cell, and HK Inno.N are developing CAR-NK (chimeric antigen receptor) cell therapies. CAR-NK, which is derived from allogeneic cells, has the advantage of being able to compen
- Company
- BESREMi lands in Big 5 Hospitals in Korea
- by Eo, Yun-Ho Mar 17, 2025 05:59am
- BESREMi, a new drug for polycythemia vera, has been approved for prescription at tertiary hospitals in Korea. According to the industry sources, PharmaEssentia Korea's BESREMi (ropeginterferon&160;alfa-2b, a treatment for polycythemia vera, has recently passed the Drug Committees (DCs) of the Big 5 medical institutions in Korea, includin
- Company
- Radioligand 'Pluvicto' available at tertiary gen hospitals
- by Eo, Yun-Ho Mar 17, 2025 05:59am
- 'Pluvicto,' a new drug for prostate cancer, is now available for prescription at tertiary general hospitals. According to industry sources, Novartis Korea's targeted radioligand therapy, Pluvicto (Lutetium vipivotide tetraxetan), has passed the drug committees (DC) of the 'Big 5' hospitals, including Samsung Medical Center, Asan Medical C
- Company
- Lotte Biologics signs MOU with Asimov for CDMO business
- by Whang, byung-woo Mar 14, 2025 05:57am
- Lotte Biologics (CEO: James Park) announced on the 13th that it has signed a memorandum of understanding (MOU) with Asimov, based in Boston, Massachusetts, for collaboration in the contract development and manufacturing organization (CDMO) business. Through the agreement, Lotte Biologics will provide services covering the entire process f
- Company
- "More BTK inhibitor options for MCL, Brukinsa as alt choice"
- by Whang, byung-woo Mar 14, 2025 05:57am
- Changes to the treatment settings have been brought to Mantle cell lymphoma (MCL) with the launching of new treatments like BTK inhibitors and implementing reimbursement. As the 2nd-generation BTK inhibitors are introduced, treatment options have been broadened for relapsed or refractory MCL, where treatment options have been limited to 1st-g
- Company
- Takadea's metastatic colorectal cancer drug Fruzqla wins nod
- by Whang, byung-woo Mar 14, 2025 05:57am
- Takeda Pharmaceutical Korea announced on March 13 that its metastatic colorectal cancer treatment, Fruzqla (fruquintinib), received domestic marketing approval from the Ministry of Food and Drug Safety (MFDS) on March 6. The efficacy·effectiveness of Fruzqla has been demonstrated for adult patients with metastatic colorectal cancer who