- Company
- Domestic approval application filed for 'Voranigo' in KOR
- by Eo, Yun-Ho Feb 27, 2025 05:57am
- New drug 'Voranigo' for malignant brain tumors has entered domestic approval procedure. According to the industry sources, Servier Korea has recently submitted approval application to the Ministry of Food and Drug Safety (MFDS) for Voranigo (vorasidenib). In Sepetember last year, this drug was designated an orphan drug in South Korea.
- Company
- Nexium challenged by P-CAB…attempts at regaining sales
- by Whang, byung-woo Feb 27, 2025 05:57am
- Proton pump inhibitors (PPIs) are being challenged by emerging P-CAB drugs. Therefore, Nexium's company is strengthening strategic partnerships to maintain Nexium's market position. The company continues partnering with Ildong Pharmaceutical and plans to continue collaboration based on its years of prescription experience. AstraZeneca
- Company
- Chong Kun Dang strengthens its oncology business
- by Kim, Jin-Gu Feb 27, 2025 05:57am
- Chong Kun Dang is tightening the reins on its oncology business. It recently acquired the right for the liver cancer drugs ‘Nexavar (sorafenib)' and ‘Stivarga (regorafenib)' and neutropenia drug ‘Neulapeg (pegfilgrastim)' in the recent month. The company expects the drugs to create synergy with its existing pipeline of anti-cancer dru
- Company
- LigaChem Bio expands partnership with Wuxi XDC
- by Cha, Jihyun Feb 27, 2025 05:57am
- LigaChem Biosciences announced on the 26th that it has signed an expanded memorandum of understanding (MOU) with Wuxi XDC to develop a next-generation antibody-drug conjugate (ADC) drug. This agreement expands the collaboration from clinical sample production to research and development in addition to the 2021 agreement that had been made be
- Company
- Dongwha makes record sales record for 4 consecutive years
- by Kim, Jin-Gu Feb 26, 2025 06:30am
- Dongwha Pharmaceutical has broken its sales record for 4 consecutive years. The company's active overseas investment and entry into new businesses are cited as the driving forces behind the record sales streak. In particular, the Vietnamese pharmacy chain it acquired in 2023 has contributed greatly to the expansion of the company's business.
- Company
- SK Bioscience will start a global trial for its mRNA vaccine
- by Cha, Jihyun Feb 26, 2025 06:29am
- SK Bioscience's messenger ribonucleic acid (mRNA) vaccine has entered full-scale clinical trials. SK Bioscience announced on the 25th that it has begun Phase I/II global clinical trials of GBP560, a Japanese encephalitis mRNA vaccine candidate. The Phase I/II clinical trial will involve 402 healthy adults living in Australia and New Ze
- Company
- Celltrion tops KRW 1T in 2024 sales
- by Chon, Seung-Hyun Feb 26, 2025 06:29am
- Celltrion's sales and operating profit are reported to be the highest in history due to expanded sales of biosimilars. Its sales exceeded KRW 1 trillion in both North American and European markets. According to the Financial Supervisory Service (FSS), Celltrion's operating profit for last year was KRW 1.2110 trillion, up 89.7% from the previo
- Company
- Samsung Bioepis launches Stelara biosimilar Pyzchiva in US
- by Whang, byung-woo Feb 26, 2025 06:29am
- Samsung Bioepis announced on the 25th that its autoimmune disease treatment Pyzchiva (ustekinumab) has been launched in the US through its marketing partner Sandoz. Stelara’s biosimilar Pyzchiva is Janssen’s treatment for autoimmune diseases such as psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis. Stelara’s a
- Company
- Celltrion’s Actemra biosimilar Avtozma receives EU approval
- by Chon, Seung-Hyun Feb 25, 2025 05:56am
- Celltrion announced on the 24th that it has received approval from the European Commission (EC) for the marketing authorization of its ‘Avtozma, a biosimilar of the autoimmune disease treatment 'Actemra (tocilizumab).’ The final approval was granted 2 months after receiving a recommendation for approval from the Committee for Medicinal P
- Company
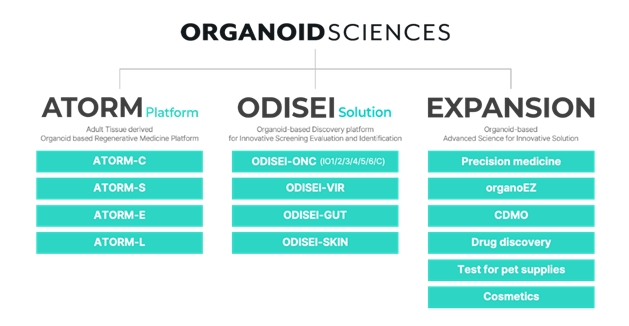
- Antibody drugs, AI, and platform companies go public
- by Cha, Jihyun Feb 25, 2025 05:56am
- Domestic pharmaceutical, bio, and healthcare companies are floating IPOs one after another. Organoid Sciences is the first to challenge the market with its &160;gap technology. IntoCell plans to submit a securities report as early as next month. Companies in various fields, including biotech companies that develop new drugs, medical artificial