- Company
- KDA ‘Concern on use of SGLT2 inhibitors for weight loss'
- by Whang, byung-woo Jan 31, 2025 05:56am
- As interest in weight loss has increased with the advent of new GLP-1 analogs, experts have warned of the risk of overuse of SGLT2 inhibitors for weight loss or cosmetic purposes. The Korean Diabetes Association has recently issued a series of statements on the safe use of SGLT2 inhibitors for both experts and the general public. As the nu
- Company
- 3 variables affecting the growth of 'Entresto'
- by Kim, Jin-Gu Jan 31, 2025 05:55am
- Novartis' chronic heart failure treatment 'Entresto (sacubitril/valsartan)' has maintained high growth, with prescription sales exceeding KRW 70 billion last year. Analysis suggests that expanded indications in 2022 and 2023 have led to skyrocketed growth. However, it is unknown whether this growth rate will continue this year. Numerous
- Company
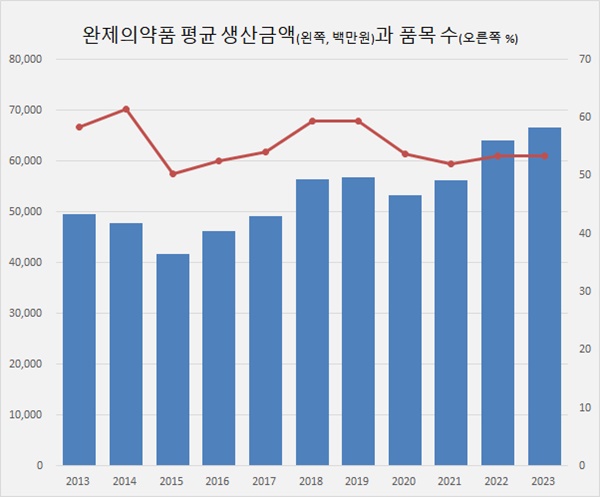
- Companies produced KRW 66.7B finished drugs a year
- by Chon, Seung-Hyun Jan 24, 2025 05:52am
- The average production performance of pharmaceutical companies has been gradually increasing. As the scale of the pharmaceutical industry grew, the average production value of both finished drugs and raw materials also continued to grow. For both finished drugs and raw materials, the share of small companies with annual production of less than K
- Company
- Will long-acting HIV treatment be included in the reimb list
- by Eo, Yun-Ho Jan 24, 2025 05:52am
- The long-acting HIV treatment 'vocabria+rekambys' gets attention whether it will be included in the National Health Insurance reimbursement list, two years after receiving approval in South Korea. According to industry sources, GSK Korea and Janssen Korea have recently entered drug price negotiations for their new HIV drugs, vocabria (cab
- Company
- The era of compound management for obesity
- by Whang, byung-woo Jan 23, 2025 05:54am
- Wegovy (semaglutide), an obesity drug hailed as a game-changer, is seeking synergy with its cardiovascular benefits in addition to weight loss. Experts say that the obesity treatment paradigm is already evolving beyond weight loss to overall health care. In the future, it will be a challenge to improve access through expert review and reimbur
- Company
- MET inhibitor Tepmetko enters last stage to reimb in KOR
- by Eo, Yun-Ho Jan 23, 2025 05:54am
- MET-targeted anticancer drug ‘Tepmetko’ has entered the final gateway to insurance reimbursement nearly 3 years after its domestic approval. According to industry sources, Merck Korea has recently started negotiating drug prices with the National Health Insurance Service for its Tepmetko (tepotinib), a treatment for locally advanced or
- Company
- "C-Trelin proven effective for SCD treatment…reimb needed"
- by Lee, Seok-Jun Jan 22, 2025 05:55am
- There is currently no standard therapy available for 'spinocerebellar degeneration (SCD).' Doctors write prescriptions based on a patient's condition, but there are still unmet needs in 'SCD treatment.' Patients affected by disease likewise. SCD is a degenerative disease affecting the cerebellum or spinal cord due to various underlying cau
- Company
- "Foreign drug price re-evaluation, unfair direct comparison"
- by Kim, Jin-Gu Jan 22, 2025 05:54am
- Yunhong Noh, President of the Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA), has criticized the government's re-evaluation of foreign drug price comparison. Noh points out that making a simple drug price comparison poses problem despite differences in socioeconomic circumstances and healthcare systems between South
- Company
- Leqembi may be prescribed in general hospitals in Korea
- by Eo, Yun-Ho Jan 22, 2025 05:54am
- The new Alzheimer’s drug Leqembi is landing in general hospitals in Korea According to industry sources, Leqembi (lecanemab) has passed the drug committees (DCs) of top tertiary hospitals in Korea, such as Samsung Medical Center, Seoul Asan Medical Center, and Sinchon Severance Hospital, as well as medical institutions such as Busan Paik
- Company
- HK Inno.N and Roche will co-promote Avastin
- by Chon, Seung-Hyun Jan 22, 2025 05:54am
- HK Inno.N announced on the 21st that it has signed a co-promotion agreement with Roche Korea for Roche’s targeted anticancer drug Avastin (bevacizumab). Avastin is an anticancer drug indicated for the treatment of metastatic colorectal cancer, metastatic breast cancer, non-small cell lung cancer, advanced or metastatic renal cell carcin