- Company
- Astellas 'Xtandi' offers high efficacy with low side effects
- by Whang, byung-woo Jan 21, 2025 05:54am
- As more treatment options are covered by reimbursement, concerns about 'how' best to treat prostate cancer are increasing. Given the increasing number of prostate cancer patients increases each year, it is important to discuss which options to provide based on the patient's condition. The specialist in the field, Dr. Hong Koo Ha, Profes
- Company
- Darzalex is granted reimb extension in Korea
- by Moon, sung-ho Jan 21, 2025 05:54am
- Multiple myeloma drug Darzalex (daratumumab) will enter the clinical field next month after successfully expanding its coverage. As new drugs such as bispecific antibody-based therapies are becoming the last treatment option in Korea, Darzalex’s success in expanding coverage has raised the prospect that combination therapy could emerge as th
- Company
- Only half of the multiple myeloma drugs reimb in KOR
- by Eo, Yun-Ho Jan 20, 2025 05:54am
- Despite the increased number of treatment options, patient access to those multiple myeloma drug options has not changed much. Multiple myeloma remains an incurable disease, but in the past, survival rates were very low due to limited treatment options. In recent years, however, innovative treatment options such as monoclonal antibodie
- Company
- Ziihera receives orphan drug designation in Korea
- by Eo, Yun-Ho Jan 20, 2025 05:54am
- The first HER2 bispecific antibody drug Ziihera has received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced so through the first orphan drug designation in the new year. Its specific indication is for the treatment of adult patients with previously treated unresectable locally advanc
- Company
- [Reporter' View] Results from J.P. Morgan Conference
- by Lee, Seok-Jun Jan 20, 2025 05:53am
- The Annual J.P. Morgan Healthcare Conference (hereafter J.P. Morgan Conference) has ended. It is the largest funding event in the pharmaceutical and biotech industry and is held in January every year. Many Korean companies have also participated in the event, to the extent that many key R&D people in the Korean pharmaceutical and biotech ind
- Company
- Expanded reimb for 'Zejula'
- by Son, Hyung Min Jan 17, 2025 05:53am
- Following the reimbursement expansion of Zejula for ovarian cancer, patient access to treatment has been improved. Experts suggest that Zejula will be more widely used in clinical practices based on demonstrated benefits in efficacy and safety in long-term treatment studies. On January 16, Takeda Pharmaceutical Korea held a press conferen
- Company
- Ultomiris is reimbursed for aHUS in Korea
- by Son, Hyung Min Jan 16, 2025 06:14am
- Ultomiris has been reimbursed in Korea for atypical hemolytic uremic syndrome (aHUS) since January. While experts have welcomed the news of its reimbursement, they have also raised the need for systematic improvements to the stringent conditions for reimbursement, including the preliminary review requirement. On the 10th, AstraZeneca Kore
- Company
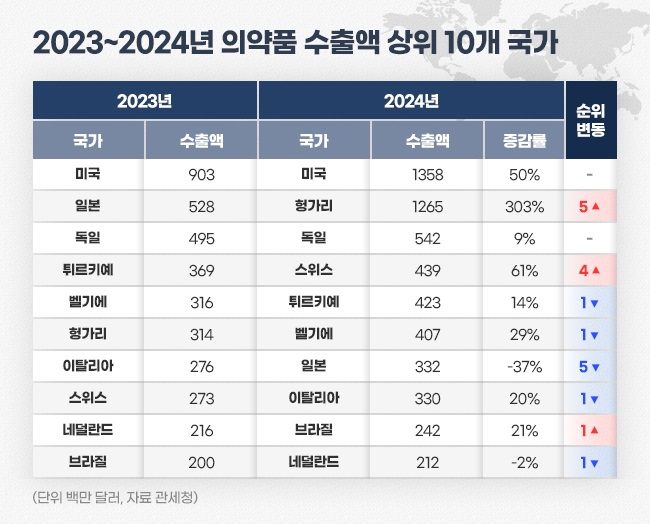
- Last year's pharma export sales recorded KRW 11T
- by Kim, Jin-Gu Jan 16, 2025 06:14am
- Last year's export sales of Korea-made pharmaceuticals amounted to US$ 7.5 billion (approximately KRW 11 trillion), up 29% from the previous year. Analysis indicates that a significant increase in biopharmaceutical exports of Samsung Biologics and Celltrion has contributed to the growth. The largest exported country was the United State
- Company
- Elahere receives orphan drug designation in Korea
- by Eo, Yun-Ho Jan 16, 2025 06:13am
- The new ovarian cancer drug Elahere has received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the designation through the first orphan drug designation announcement of the new year. Specifically, the drug is indicated as monotherapy in adult patients with folate receptor-alpha (FR
- Company
- Companies develop new drugs for Fabry disease
- by Son, Hyung Min Jan 16, 2025 06:13am
- Domestic and foreign pharmaceutical companies are speeding up the development of new drugs for the rare Fabry disease. They aim to develop treatments that improve not only efficacy and safety but also administration methods compared to existing treatments. According to industry sources on the 15th, the Ministry of Food and Drug Safety rec