- Company
- JW Pharma's hemophilia assay for patients using 'Hemlibra'
- by Kim, Jin-Gu Jan 16, 2025 06:13am
- JW Pharmaceutical announced on January 15 that its chromogenic assay designed to assess the severity of 'hemophilia' in patients using 'Hemlibra' has been commercialized in South Korea. The Ministry of Health and Welfare (MOHW) has newly established a non-reimbursable CSA testing criterion, effective January 1. CSA test is used to m
- Company
- China-made new drugs actively enter the global market
- by Son, Hyung Min Jan 16, 2025 06:13am
- Many Chinese pharmaceutical and biotech companies are demonstrating results from their new anti-cancer drug development in the global market. Companies like Jiangsu Hengrui Pharmaceuticals and BeiGene have obtained approvals for their immunotherapy and targeted anti-cancer agents from the regulatory authorities globally, including South Korea, t
- Company
- ‘Global healthcare M&A to recover this year... AI-China’
- by Cha, Jihyun Jan 15, 2025 05:53am
- Artificial intelligence (AI) and China are expected to be the keywords for mergers and acquisitions (M&A) in the global bio and healthcare sector this year. On the 14th, KoreaBIO disclosed a report by global accounting consulting firm Ernst & Young (EY) that contained the prospects above. According to the report, 130 M&A deals were made in
- Company
- Cholangiocarcinoma·AML targeted anticancer drug 'Tibsovo'
- by Eo, Yun-Ho Jan 15, 2025 05:53am
- 'Tibsovo,' a targeted anticancer drug for cholangiocarcinoma and acute myeloid leukemia (AML), is now available for prescription in general hospitals. According to industry sources, Servier's Tibsovo (ivosidenib), a drug targeting the isocitrate Dehydrogenase 1 (IDH-1) gene mutation, has passed the drug committees (DC) of the 'Big 5' tert
- Company
- Samsung Biologics signs largest CMO deal…KRW 2 Trillion
- by Cha, Jihyun Jan 15, 2025 05:52am
- Samsung Biologics announced on the 14th that it has signed a contract manufacturing organization (CMO) agreement worth USD 1.41 billion (approximately KRW 2.74 trillion) with a Europe-based pharmaceutical company. This is the largest contract in Samsung Biologics' history. It represents 40% of the total order value of KRW 5.4035 trillion
- Company
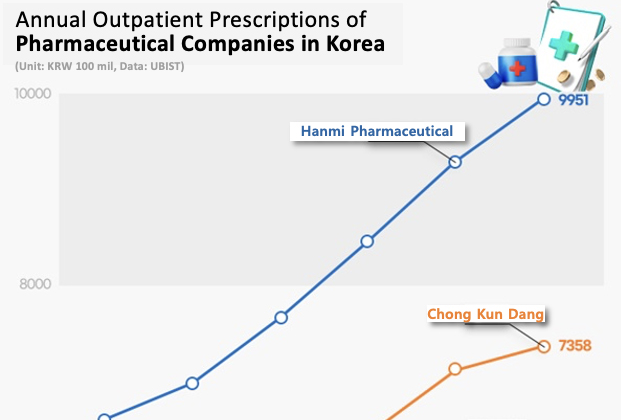
- Hanmi leads the prescription market for 7 consecutive years
- by Chon, Seung-Hyun Jan 15, 2025 05:52am
- Hanmi Pharmaceutical has led the domestic outpatient prescription market for 7 consecutive years. The company has solidified its lead in the market fueled by the strong performance of the new combination drugs it developed with its R&D capabilities. Large pharmaceutical companies such as Chong Kun Dang, Daewoong Pharmaceutical, Yuhan Corp, HK In
- Company
- 'Leclaza·Rybrevant comb therapy' wins nod in KOR
- by Son, Hyung Min Jan 14, 2025 05:56am
- The approval of Leclaza plus Rybrevant combination therapy in South Korea has expanded treatment options for patients with non-small cell lung cancer (NSCLC). As Leclaza combination therapy has shown a positive overall survival (OS) result, it is highly likely to be the first-line standard therapy for treating EGFR-positive NSCLC. Accordin
- Company
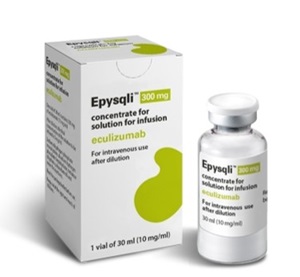
- Samsung Bioepis partners with Teva for Soliris biosimilar
- by Cha, Jihyun Jan 14, 2025 05:56am
- Samsung Bioepis announced on the 12th that it has signed a commercialization partnership agreement with the multinational pharmaceutical company Teva Pharmaceutical Industries to launch Epysqli, a biosimilar of the rare disease treatment Soliris (eculizumab), in the U.S. market. Epysqli is the first hematology biosimilar developed by Sam
- Company
- Will biotech companies continue 'K-Bio' success?
- by Moon, sung-ho Jan 13, 2025 05:53am
- Korean pharmaceutical and biotech companies have successfully outlicensed new drugs in various fields, including cancer, autoimmune disease, and Alzheimer's disease. However, compared to the year before, analysis suggests that the number of out-licensed cases decreased. Despite such a slowdown, companies focusing on global new drug developmen
- Company
- Samsung Bioepis to indirectly sell Soliris biosimilar in US
- by Cha, Jihyun Jan 13, 2025 05:53am
- Samsung Bioepis has signed a partnership agreement with the multinational pharmaceutical company Teva Pharmaceuticals to commercialize biosimilars of rare disease treatments in the United States. Samsung Bioepis will launch the biosimilar through Teva in the U.S. market in the first half of this year. Unlike in Europe, where Samsung Bioepis h