- Company
- Wegovy can be prescribed in tertiary hospitals in KOR
- by Eo, Yun-Ho Jan 13, 2025 05:53am
- The obesity drug ‘Wegovy’ can now be prescribed in tertiary hospitals in Korea. According to industry sources, Novo Nordisk Korea's Wegovy (semaglutide) has passed the drug committees (DCs) of Korrea’s “Big 5 medical institutions,” including Samsung Medical Center, Seoul National University Hospital, and Sinchon Severance Hospital.
- Company
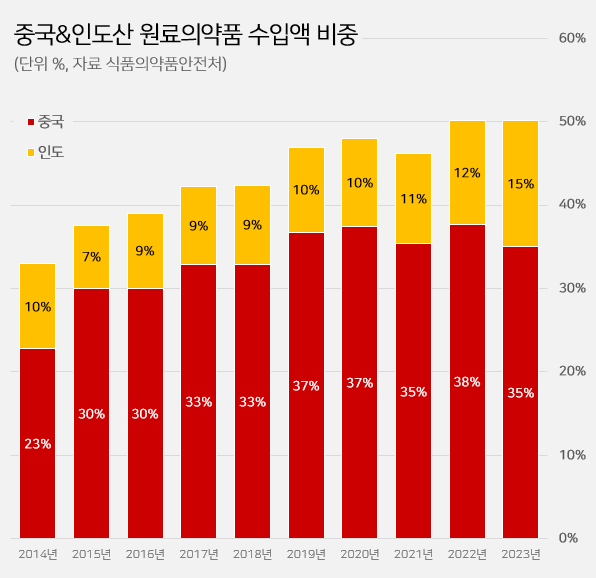
- Imported API from China·India reaches 50%
- by Kim, Jin-Gu Jan 13, 2025 05:53am
- It has been reported that half of the active ingredients (API) imported to South Korea are produced in China and India. The percentage of imported active ingredients originating from China·India surpassed 50% in two consecutive years. The percentage of imported API from India has robustly increased recently. Indian API imports were below 10
- Company
- Sanofi’s Hexaxim is included in NIP from this year
- by Whang, byung-woo Jan 10, 2025 05:52am
- Sanofi announced on the 9th that Hexaxim, its hexavalent combination vaccine for infants, has been included in Korea’s National Immunization Program (NIP). With its introduction to the NIP, Hexaxim can now be administered free of charge at designated medical institutions under the National Immunization Program for Children. As the fir
- Company
- Hexaxim may be administered in general hospitals in Korea
- by Eo, Yun-Ho Jan 10, 2025 05:52am
- Hexaxim, a hexavalent combination vaccine for infants that was included in the National Immunization Program, may now be administered in general hospitals. According to industry sources, Sanofi Korea's Hexaxim prefilled syringe has now passed the drug committees (DCs) of 17 medical institutions nationwide, including Seoul National Univers
- Company
- Lilly Korea releases Ebglyss for atopic dermatitis in Korea
- by Whang, byung-woo Jan 10, 2025 05:52am
- Lilly Korea announced on the 9th that it had launched Ebglyss (lebrikizumab) in Korea for the treatment of moderate-to-severe atopic dermatitis.&160; Ebglyss is a novel biologic agent that selectively blocks cytokine interleukin (IL)-13, a major cause of atopic dermatitis. It was approved by the Ministry of Food and Drug Safety in Au
- Company
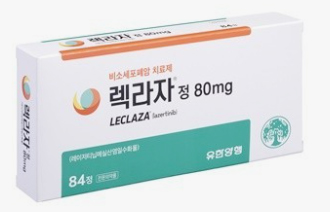
- Leclaza’s new trial data shows improved OS
- by Son, Hyung Min Jan 9, 2025 05:57am
- The Leclaza plus Rybrevant combination achieved statistically significant overall survival (OS) results. Johnson & Johnson expects Leclaza plus Rybrevant to extend OS by more than a year compared to Tagrisso monotherapy. The positive OS outcome for the combination strengthens its potential to become the first-line standard of care for EGFR-p
- Company
- Vabysmo approved for retinal vein occlusion macular edema
- by Whang, byung-woo Jan 9, 2025 05:56am
- Roche Korea announced on the 8th that Vabysmo has been approved by the Ministry of Food and Drug Safety (MFDS) for the treatment of visual impairment due to macular edema secondary to retinal vein occlusion. With the approval, Vabysmo is now approved for 3 indications in Korea, including as a treatment for ▲neovascular (wet) age-relate
- Company
- Krazati receives orphan drug designation in Korea
- by Eo, Yun-Ho Jan 9, 2025 05:56am
- The second KRAS inhibitor 'Krazati' has been designated as an orphan drug in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the news through the first orphan drug designation announcement of the new year. Specifically, Krazati is indicated for 'locally advanced or metastatic non-small cell lung cancer (NSCLC) wit
- Company
- Wegovy vs Mounjaro in KOR…who's the winner?
- by Moon, sung-ho Jan 9, 2025 05:56am
- As more people are living with obesity in the world, obesity treatment is gaining popularity. According to the World Obesity Federation report, more than half of the world's population in 2035, 10 years from now, will be categorized as overweight or obese. South Korea is projected to have a similar rate. At the end of last year, Novo Nordisk'
- Company
- Sales of Flu drug Tamiflu did not fare so well last year
- by Nho, Byung Chul Jan 8, 2025 05:53am
- The oseltamivir-based flu treatment market, commonly represented by Tamiflu, is on a vertical decline after peaking in sales in 2023. The market for related preparations was valued at KRW 35.6 billion in 2023, the largest in 5 years. By 3Q 2024, the oseltamivir market posted sales of KRW 6.2 billion; even when the sales of the fourth q