- Company
- K-Bio to showcase CDMO·new drugs at the JP Morgan
- by Son, Hyung Min Jan 2, 2025 06:10am
- The Korean pharmaceutical and biotech industry is set to participate in the new year's first global event, the JP Morgan Healthcare Conference. Attention has been drawn to what the Korean pharmaceutical and biotech industry will accomplish during the event, which will include discussions of blockbuster technology transfers and partnering a
- Company
- Yuhan Corp will distribute Canesten·Bepanthen
- by Nho, Byung Chul Dec 30, 2024 05:57am
- Yuhan Corp has been selected as the new distribution partner for Bayer’s Canesten and Bepanthen. According to industry sources, Bayer Korea recently terminated its 10-year co-promotion agreement with Ildong Pharmaceutical and signed a new distribution agreement with Yuhan Corp. Under the new agreement, Yuhan Corp will take over the
- Company
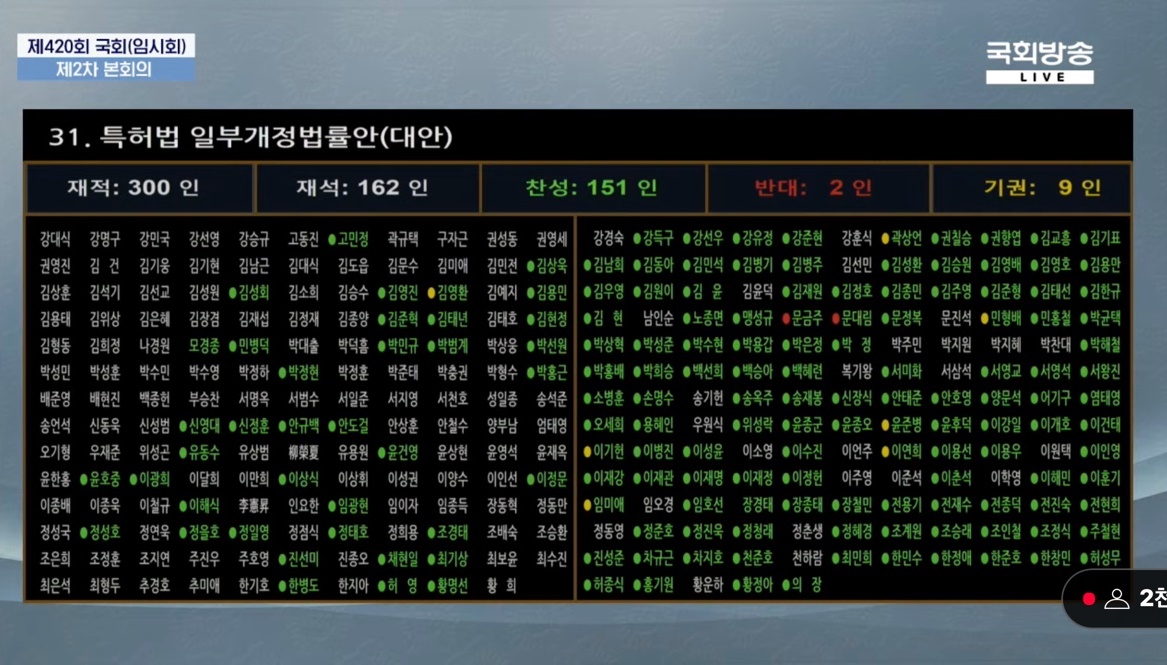
- Companies show mixed responses to patent term limitation law
- by Kim, Jin-Gu Dec 30, 2024 05:57am
- A bill to amend the Patent Act to limit the patent period for new drugs has passed the plenary session of the National Assembly. The amendment will limit the upper limit of the remaining patent term to a maximum of 14 years from the time a new drug is approved and allow only one of several patents registered for a single drug to be extended.
- Company
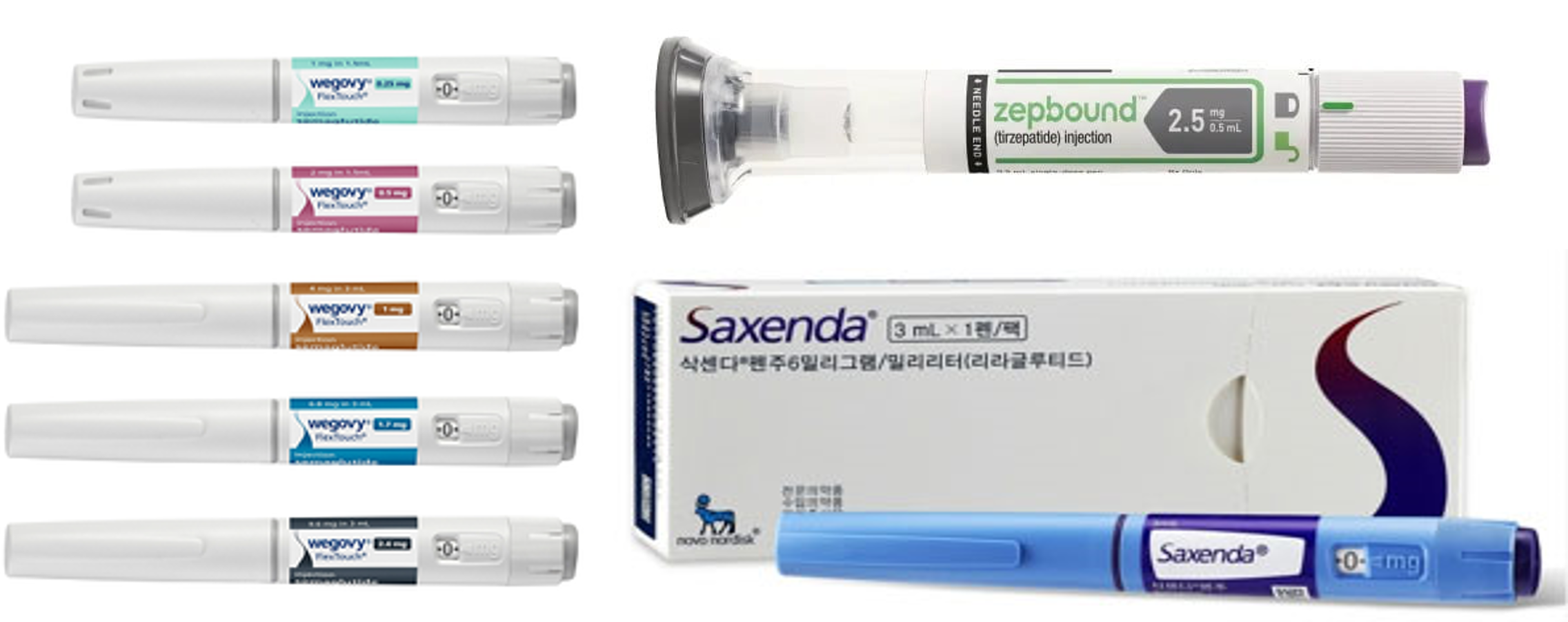
- Generic drugs mkt heat up as GLP-1 obesity meds demand soar
- by Son, Hyung Min Dec 30, 2024 05:57am
- It has been reported that not only new drugs but also generics have joined the competition in the market for glucagon-like peptide-1 (GLP-1)- containing treatment for diabetes and obesity. In the United States, generic versions of liraglutide, which is an active ingredient of the diabetes obesity·treatments Victoza·Saxenda that are GLP-1 recep
- Company
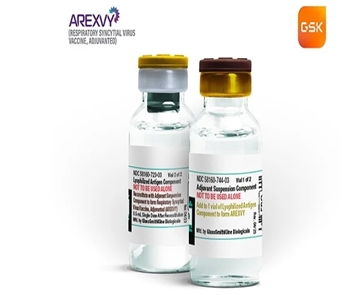
- First RSV vaccine Arexvy Inj lands in Korea
- by Whang, byung-woo Dec 27, 2024 05:56am
- Arexvy, GSK's global sales driver as a No. 1 adult RSV vaccine, is set to launch in Korea. GSK Korea's Respiratory Syncytial Virus (RSV) vaccine Arexvy was recently approved by the Ministry of Food and Drug Safety (MFDS) for the prevention of lower respiratory tract disease (LRTD) caused by RSV in adults aged 60 years and older. After
- Company
- SK Bioscience and Sanofi sign contract to co-develop
- by Cha, Jihyun Dec 26, 2024 05:51am
- SK Bioscience has extended the scope of vaccine development in collaboration with the global pharmaceutical company Sanofi. The company aims to advance the pneumococcal conjugate vaccine currently being developed to the next-generation vaccine. SK Bioscience and Sanofi announced on December 23 that they have signed an agreement to co-deve
- Company
- Daiichi Sankyo to build manufacturing facilities in China
- by Kim, Jin-Gu Dec 26, 2024 05:50am
- Daiichi Sankyo will build a manufacturing facility in China for its ADC (antibody-drug conjugate) anticancer drug Enhertu. The plant, which will be built in Shanghai, is scheduled to be completed in 2030, and its products will be supplied to China. According to KoreaBIO, Daiichi Sankyo recently announced plans to build a manufacturing fac
- Company
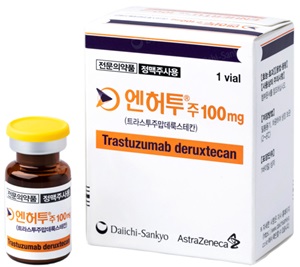
- K-Bios seek drugs to be used in combination with ADCs
- by Son, Hyung Min Dec 26, 2024 05:50am
- The domestic pharmaceutical and bio-industry are changing clinical trial protocols and confirming the possibility of their use in combination therapy with antibody-drug conjugates (ADCs). In particular, a growing number of companies are trying to use their drugs in combination with Enterhu, which has shown an effect across solid cancers For e
- Company
- Losartan prescription market has grown 15% in 3 years
- by Chon, Seung-Hyun Dec 26, 2024 05:50am
- The prescription market for the antihypertensive drug losartan has shown an upward trend. Its market plummeted in 2021 following the detection of excess impurities in all losartan products but has since recovered obviously. Prescriptions of both single and combination losartan drugs have risen over 10% from three years ago. Analysts say the recu
- Company
- Soyun Oh appointed to head Organon Malaysia
- by Whang, byung-woo Dec 24, 2024 06:22am
- Organon Korea announced today that Soyun Oh, who currently heads the company’s Sales and Customer Department, has been appointed Country Lead for Organon Malaysia, effective January 1, 2025. With more than 26 years of experience in the industry, Oh has been with Organon since its inception in Korea and has successfully led the company'