- Company
- Mounjaro aims for 'diabetes·obesity' synergy
- by Whang, byung-woo Nov 19, 2024 06:12am
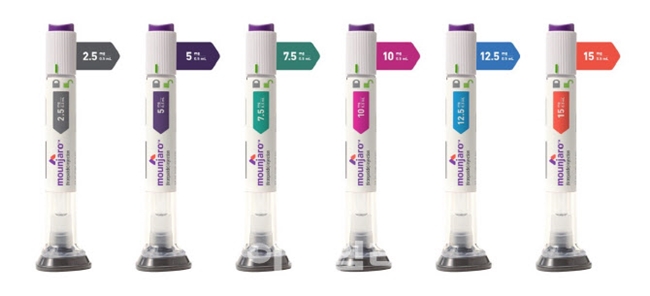
- Eli Lily's Mounjaro (tirzepatide), the first once-weekly GIP·GLP-1 receptor agonist, will enter the market with its diabetes and obesity indications. Unlike already launched Wegovy (semaglutide), Mounjaro has two indications: diabetes and obesity. Experts anticipate that Mounjaro's sales strategy will be focused on diabetes treatment a
- Company
- Academia pushes for Tagrisso’s reimbursement expansion
- by Moon, sung-ho Nov 19, 2024 06:12am
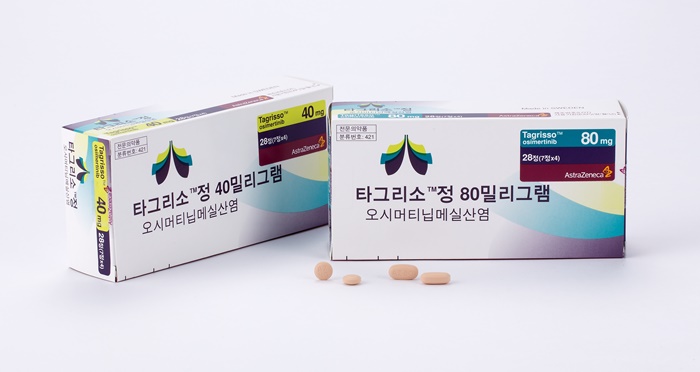
- Less than a year after AstraZeneca's lung cancer drug Tagrisso (osimertinib) was granted expanded reimbursement coverage, another round of discussions on its further expansion has begun, drawing attention to its background. As the expansion request came from the medical community, not pharmaceutical companies, it will be interesting to see i
- Company
- Premium meningitis vaccine Bexsero lands in hospitals in KOR
- by Eo, Yun-Ho Nov 18, 2024 05:49am
- The next-generation meningococcal vaccine, ‘Bexsero’ can now be prescribed in general hospitals in Korea. According to industry sources, GSK Korea’s Bexsero, the first meningococcal B vaccine introduced to Korea, has passed the drug committees (DCs) of the Big 5 tertiary hospitals, including Samsung Medical Center, Seoul National Uni
- Company
- 'Tevimbra' can be prescribed at general hospitals
- by Eo, Yun-Ho Nov 18, 2024 05:49am
- 'Tevimbra,' a type of immune checkpoint inhibitor, is becoming more widely available for prescription at general hospitals. According to industry sources, BeiGene's PD-1 inhibitor Tevimbra (tislelizumab), which has applied for reimbursement for its esophageal cancer indication, has passed the drug committees (DC) of tertiary general hospi
- Company
- Demand rises for reform of the cancer drug reimb system
- by Moon, sung-ho Nov 18, 2024 05:49am
- With the presence of new anticancer drugs increasing in the clinical field, the pharmaceutical industry has been demanding the authorities apply a new reimbursement model in Korea. In addition to the existing immuno-oncology drugs, the emergence of antibody-drug conjugates (ADCs), which have proven effective in various cancers, has led to cal
- Company
- Six reimbursement revaluations fail in 4 years
- by Chon, Seung-Hyun Nov 15, 2024 05:49am
- Six items have been removed from the reimbursement list after reimbursement reevaluations during the past 4 years. Silymarin bilberry fruit dried powder, followed by itopride, failed the reimbursement reevaluations. Streptokinase - streptodornase, oxiracetam, and acetylcarnitine were also removed due to reimbursement reevaluation failures. Pharm
- Company
- Leqvio with 'twice-yearly treatment' set for mkt
- by Whang, byung-woo Nov 15, 2024 05:49am
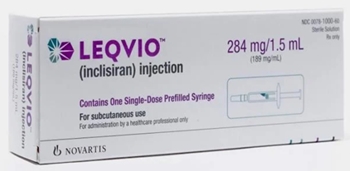
- Leqvio is set to challenge the market with its superior drug tolerance, administered twice yearly, compared to existing treatments. According to the pharmaceutical industry on November 15, Novartis Korea launched the siRNA therapy Leqvio (inclisiran) on November 11. Lecvio is the first-in-class siRNA drug approved in South Korea. It is
- Company
- Hanmi's new bio drug 'Rolvedon' sales 88%↑ in the US mkt
- by Son, Hyung Min Nov 15, 2024 05:49am
- The U.S. sales of Rolvedon (Korean product name: Rolontis) have substantially increased. Hanmi Pharmaceutical's U.S. partnering company, Assertio, plans to expand market share by securing Rolvedon's indication for the same-day administration. According to Assertio on Novermber 14, Rolveron's sales in the U.S. market in Q3 were US$ 15 million
- Company
- Lee Hyun-ju named as the representative of ZP Therapeutics
- by Eo, Yun-Ho Nov 14, 2024 05:52am
- Lee Hyun-ju (48), ex-Daiichi Sankyo Korea's Oncology Business Franchise Head, was appointed as the new representative of ZP Therapeutics Korea. Industry sources said Zuellig Pharma has recruited Lee Hyun-ju as the new head of ZP Therapeutics Korea, Zuellig Pharma's commercial services corporation. Lee holds a degree from Sungkyunkwan
- Company
- Celltrion anticipates Trump admin will bring positive shift
- by Whang, byung-woo Nov 14, 2024 05:51am
- Celltrion anticipates that President-elect Donald Trump's second administration in the United States will positively affect the company's biosimilars and Contract Development and Management Operations (CDMO) services. On November 12, Celltrion presented stockholders with the potential impact on the business under the title 'The Business Im