- Company
- ‘Access to bispecific antibody Columvi should be improved’
- by Son, Hyung Min Nov 14, 2024 05:51am
- “Diffuse large B-cell lymphoma (DLBCL) is a disease in which one in four patients experience relapse even after treatment. The bispecific antibody Columvi has demonstrated efficacy in relapsed patients at up to 18 months of follow-up. The clinical performance of Columvi is not just an incremental improvement over existing therapies, but a p
- Company
- Global CDMOs compete to expand ADC capacities
- by Kim, Jin-Gu Nov 14, 2024 05:51am
- Global competition is heating up in the contract development manufacturing organization (CDMO) market for antibody-drug conjugates (ADCs). Major players include Switzerland's Lonza and Samsung Biologics, the world's top two CDMOs, which are competitively expanding their manufacturing facilities. Lonza recently announced the expansion of a
- Company
- Imfinzi combo drug Imjudo can be prescribed at hospitals
- by Eo, Yun-Ho Nov 13, 2024 05:54am
- Immuno-oncology drug Imfinzi's combination partner Imjudo may now be prescribed in general hospitals in Korea. According to industry sources, AstraZeneca Korea's CTLA-4 inhibitor Imjudo (tremelimumab) has passed the drug committees (DCs) of tertiary hospitals in Korea including Seoul National University Hospital and Seoul Asan Medical Cen
- Company
- Treatment-refractory Dravet syndrome calls for new options
- by Whang, byung-woo Nov 13, 2024 05:54am
- Despite increased treatment options for the ultra-rare Dravet syndrome, there are still gaps in care that require attention. Even with the introduction of medical cannabis, cannabidiol, there are patients who do not respond to the drug, which is why improving access to new options should be considered. Dravet syndrome is a rare neuro
- Company
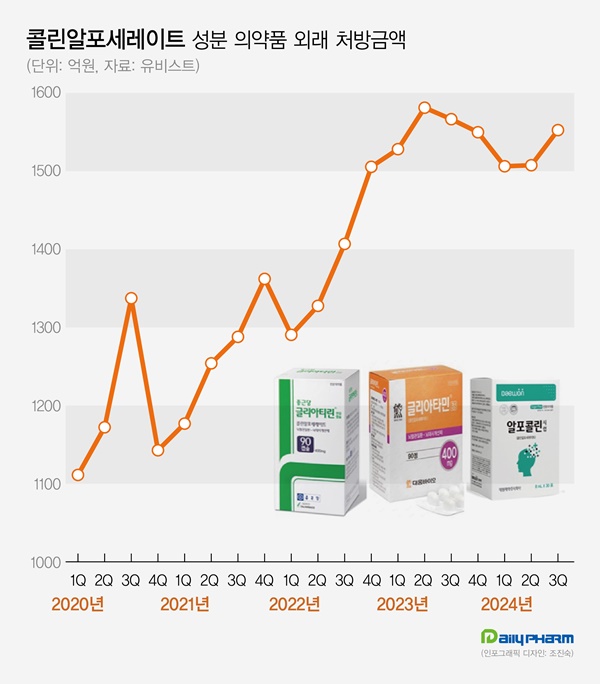
- 'Choline alfoscerate' prescription market continues to grow
- by Chon, Seung-Hyun Nov 13, 2024 05:54am
- The cognitive enhancer 'choline alfoscerate (choline products)' has expanded its presence in the prescription market. Choline products' growth slowed earlier this year but rebounded in Q3, further expanding the market size. Although a few companies withdrew from the market due to the risk of failing clinical re-evaluation, the prescription marke
- Company
- Asthma drug 'Monterizine' sales rise despite generic entries
- by Kim, Jin-Gu Nov 12, 2024 05:51am
- Hanmi's asthma treatment, 'Monterizine,' successfully expanded its prescription sales over 10% Year-over-Year (YoY), despite the release of generics. Generic drugs had been listed for reimbursement in October 2023. The analysis suggests that Monterizine's continued sales expansion is because generic prices have not been reduced and, it mainta
- Company
- 'Vabysmo' for macular deg associated RVO indication expected
- by Eo, Yun-Ho Nov 12, 2024 05:51am
- The first bispecific antibody for eye diseases, 'Vabysmo,' is soon to be approved in South Korea for its indication of treating macular degeneration associated retinal vein occlusion (RVO). According to industry sources, Roche Korea's Vabysmo (faricimab) is being reviewed by the Ministry of Food and Drug Safety (MFDS) for its indication ex
- Company
- Generic companies seek early entry into KRW 100 bil Tagrisso
- by Kim, Jin-Gu Nov 12, 2024 05:51am
- Patent challenges to Tagrisso (osimertinib), a non-small cell lung cancer treatment that posts annual sales of KRW 110 billion, are expanding.&160; The companies that have filed patent challenges seek to first evade the product patent, which expires in 2035, and then launch generics early after November 2033, when the substance patent ex
- Company
- Will Lorviqua be reimbursed as a first-line therapy in KOR?
- by Eo, Yun-Ho Nov 11, 2024 05:49am
- Whether progress will be made in the insurance reimbursement discussions for the ALK antitumor drug Lorviqua is gaining attention. The health authorities recently said they would “promptly start discussions” on the need to expand coverage of the ALK-positive NSCLC drug Lorviqua (lorlatinib) to the first-line. Lorviqua is currently in
- Company
- Companies copromote products at Hypertension Seoul 2024
- by Kim, Jin-Gu Nov 11, 2024 05:49am
- Pharmaceutical and biotech companies with hypertension drugs have gathered at the Conrad Seoul Hotel in Yeouido, Seoul on the 8th. Industry officials, who set up promotional booths at the Korean Society of Hypertension's fall conference (Hypertension Seoul 2024), distributed pamphlets to doctors at the event and introduced the features of t