- Company
- CKD’s CKD-508 receives approval to initiate P1T in the U.S.
- by Son, Hyung Min Nov 6, 2024 05:52am
- Chong Kun Dang announced on the 4th that it has received approval from the U.S. Food and Drug Administration (FDA) for the Phase I clinical trial of CKD-508, its new drug candidate for dyslipidemia. In the trial, Chong Kun Dang will confirm the safety and lipid-improving effects of CKD-508 and explore the optimal dose for a Phase II trial
- Company
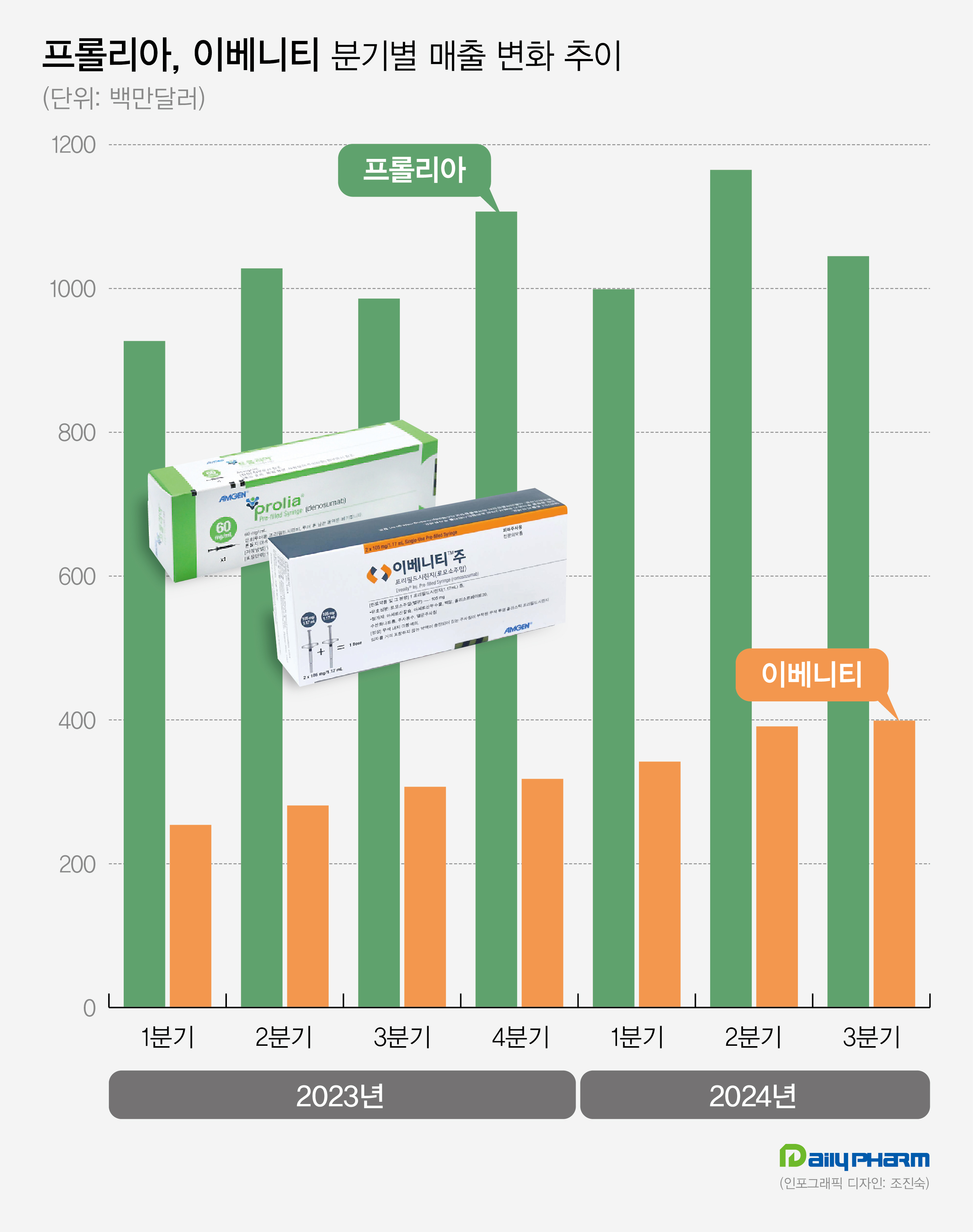
- Amgen's Prolia·Evenity generate KRW 2T in quarterly sales
- by Son, Hyung Min Nov 6, 2024 05:52am
- Amgen's Prolia and Evenity for the treatment of osteoporosis continue to show sales growth. Prolia and Evenity led Amgen's performance, generating sales of KRW 2 trillion in Q3. Analysis suggests that sequential therapy, using Evenity followed by Prolia for the treatment of osteoporosis with high-risk fractures, has been widely used, and it
- Company
- Chong Kun Dang speeds up dyslipidemia drug development
- by Son, Hyung Min Nov 5, 2024 05:46am
- &160; Chong Kun Dang is accelerating the development of new drugs for dyslipidemia. The company has received approval to initiate a second global clinical trial in 10 years for its new drug candidate 'CKD-508' since the company began its research in 2014. According to industry sources on the 4th, Chong Kun Dang recently received approva
- Company
- ‘Prevent MI recurrence through efficient LDL-C control'
- by Whang, byung-woo Nov 5, 2024 05:45am
- With the rise of metabolic diseases such as hypertension, diabetes, and hyperlipidemia increase in Korea, the prevalence of myocardial infarction and atherosclerotic cardiovascular diseases are also on the rise. The mortality rate of myocardial infarction is in the 20-30% range when it occurs for the first time, but the mortality rate increas
- Company
- New oHCM drug 'Camzyos' nearing approval for reimb in KOR
- by Eo, Yun-Ho Nov 5, 2024 05:45am
- 'Camzyos,' a new drug to treat obstructive hypertrophic cardiomyopathy (oHCM), is nearing 90% approval for insurance reimbursement listing. Sources said that BMS Pharmaceutical Korea and the National Health Insurance Service (NHIS) concluded drug pricing negotiations for Camzyos (mavacamten), a new drug for obstructive hypertrophic cardi
- Company
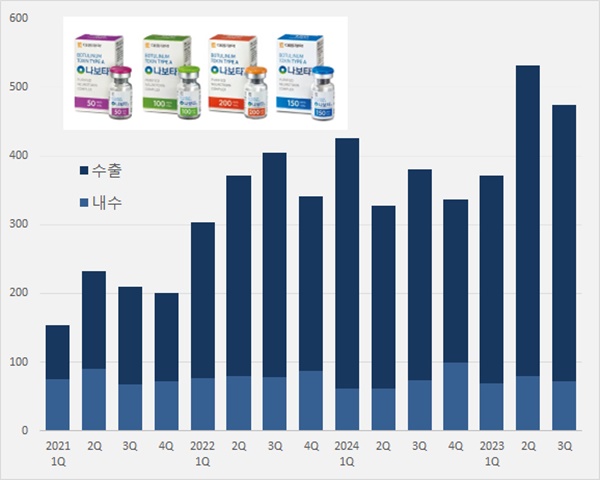
- 'Nabota' generated KRW 115.8B over 9 mths in foreign mkt
- by Chon, Seung-Hyun Nov 5, 2024 05:45am
- Daewoong's Nabota, which contains botulinum toxin, is expanding its presence in the foreign market. Its export amount surpassed KRW 100 billion up to Q3 2024. Over 80% of the overall sales were accounted for by sales generated in the foreign market. It is a cash cow export product. According to Daewoong on November 2, Nabota generated sales o
- Company
- 'SGLT2·DPP4' comb market records robust growth
- by Kim, Jin-Gu Nov 4, 2024 05:48am
- The market for combination drugs containing SGLT-2 inhibitor and DPP-4 inhibitor for treating type 2 diabetes is showing rapid growth. The industry expects the market size to expand by over KRW 30 billion this year. Products containing one or more original active ingredients are leading the market growth. Boehringer Ingelheim's 'Esglite
- Company
- Will the polycythemia vera drug Besremi be reimb in KOR?
- by Eo, Yun-Ho Nov 4, 2024 05:48am
- Whether PharmaEssentia Korea’s new drug for polycythemia vera, ‘BESREMi,’ will be listed with reimbursement in Korea is gaining attention. The drug was approved for hydroxyurea-refractory or intolerant polycythemia vera in March last year but failed to overcome the CDDC barrier in July of the same year. At that time, the CDDC dete
- Company
- K-Bios globally present immuno-oncology drugs
- by Son, Hyung Min Nov 4, 2024 05:48am
- The development achievements of the domestic pharmaceutical bio industry's immuno-oncology drugs will be presented at an overseas conference. Hanmi Pharmaceutical, GC Cell, Abion Bio, ST Cube, and Y-Biologics, among others, have completed preparations to emerge into the international stage by disclosing positive clinical trial results.&16
- Company
- Reimb of Vocabria+Rekambys for HIV gains attention
- by Eo, Yun-Ho Nov 4, 2024 05:48am
- The industry’s eyes are on whether the long-acting HIV combination therapy ‘Vocabria+Rekambys’ will be reimbursed by the end of the year in Korea. According to industry sources, GSK Korea and Janssen Korea have completed the pharmacoeconomic evaluation of their HIV drugs Vocabria (cabotegravir) and Rekambys (rilpivirine) combination