- Company
- Will Imfinzi pass CDDC review with biliary tract cancer data
- by Moon, sung-ho Sep 5, 2024 05:52am
- Biliary tract cancer is known to have the highest mortality and incidence rates in Korea in the world. It is also one of the most difficult cancers to treat in Korea due to the limited options, or ‘weapons’ available to doctors in practice. However, immuno-oncology drugs are being approved for the first-line treatment of biliary tract cance
- Company
- New lymphoma drug 'Columvi' reapplies for reimbursement
- by Eo, Yun-Ho Sep 5, 2024 05:51am
- 'Columvi,' a first-in-class bispecific antibody for lymphoma treatment, will make another attempt to be considered for reimbursement listing. According to industry sources, Roche Korea has recently submitted a reimbursement application for the CD20·CD3 bispecific antibody Diffuse Large B-Cell Lymphoma (DLBCL) treatment Columvi (glofitama
- Company
- More treatment options for rheumatoid arthritis available
- by Hwang, Byung-woo Sep 5, 2024 05:51am
- "Patients with rheumatoid arthritis differ by age, duration of illness, accompanying disease, self-injection capability, risk factor for complications, and economic status. Therefore, drug selection considering an individual's condition is crucial." Rheumatoid arthritis is caused by abnormally activated immune cells attacking joints, t
- Company
- J&J appoints Christian Rodseth as new Managing Director
- by Hwang, Byung-woo Sep 5, 2024 05:51am
- Janssen Korea, a subsidiary of Johnson and Johnson (J&J)'s pharmaceutical division in South Korea, announced on September 4th that Christian Rodseth was appointed as the new Managing Director for J&J's pharmaceutical division in North Asia as of September 2nd. As the CEO of Janssen Korea and Managing Director for J&J's pharmaceutical div
- Company
- New drug Voranigo receives orphan drug designation in Korea
- by Eo, Yun-Ho Sep 4, 2024 05:49am
- Voranigo, the first new malignant brain tumor drug introduced in 20 years, has received an orphan drug designation in Korea. The Ministry of Food and Drug Safety announced so through an official orphan drug designation notice on the 3rd. Specifically, the drug is for the treatment of IDH-mutated diffuse glioma. Voranigo can be used to
- Company
- Yuhan's investment in US subsidiary total KRW 27B over 5 yrs
- by Chon, Seung-Hyun Sep 4, 2024 05:48am
- Yuhan Corporation has invested KRW 3.9 billion additionally to the U.S.-based subsidiary of the company. The company invested KRW 27 billion over the past five years, expanding support for global entry. According to Financial Supervisory Service on September 4th, Yuhan Corporation invested KRW 3.9 billion in the first half of the year and acq
- Company
- Generic companies lastly challenge Lixiana patent
- by Kim, Jin-Gu Sep 4, 2024 05:48am
- Generic companies have been filing late patent challenges against Lixiana (edoxaban), the market leader in the direct-acting oral anticoagulants (DOACs) market. This is due to the imminent expiry of the product's formulation patent, which is 2 years away. The strategy of the patent challengers is to avoid the formulation patent and laun
- Company
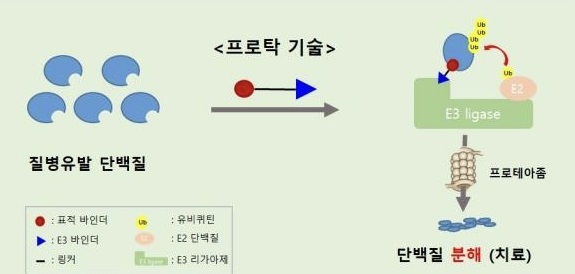
- Pharma companies jump into developing 'TPD' for cancer
- by Son, Hyung-Min Sep 3, 2024 05:53am
- The global and Korean pharmaceutical industries are focusing on securing Targeted Protein Degradation (TPD) technology, which has emerged following the success of Antibody-Drug Conjugates (ADCs). Recently, major global pharmaceutical companies such as Pfizer, Novartis, and Eli Lilly have successfully acquired TPD technologies, marking TPD as a n
- Company
- Biological drug options for psoriasis treatment increase
- by Son, Hyung-Min Sep 2, 2024 05:48am
- Domestic and foreign pharmaceutical companies are expanding psoriasis treatment options through the development of biological agents. Recently, Korea's UCB Pharma succeeded in obtaining domestic marketing authorization for a biological drug with a new mechanism of action, sparking competition in the field. Other domestic pharmaceutical and biote
- Company
- Yuhan’s Leclaza targets US mkt based on its clinical effect
- by Hwang, Byung-woo Sep 2, 2024 05:48am
- The non-small cell lung cancer drug Leclaza (lasertinib) in combination with Ryvrevant(amivantamab) has gained competitivity in the U.S., based on data presented at the World Congress on Lung Cancer (WCLC 2024). Additional data on Leclaza monotherapy and Leclaza in combination with Ryvrevantare is expected to be presented to further increase