- Company
- Rare disease drug Ilaris is reimbursed 9 yrs after approval
- by Hwang, Byung-woo Aug 11, 2024 04:46pm
- Expectations are rising in the field for the hereditary recurrent fever syndrome Ilaris (canakinumab), which was granted reimbursement 9 years after approval. As a treatment for an extremely rare disease with a small number of patients, its reimbursement is expected to address unmet needs in an area with no treatment option. However, due
- Company
- Yuhan acquires new drugs from biotech venture firms
- by Chon, Seung-Hyun Aug 9, 2024 05:34am
- Yuhan significantly increased its research and development (R&D) investment. Over the past five years since acquiring new drug technology from biotech venture companies, Yuhan's R&D investment size has reached its peak. This expansion of outward investment seems to be the company's outlook for securing a new portfolio. According to Yuhan on J
- Company
- SK Biopharmaceuticals records profit for 3 quarters
- by Kim, Jin-Gu Aug 9, 2024 02:55am
- SK Biopharmaceuticals succeeded in recording an operating profit for the third consecutive quarter. SK Biopharmaceuticals, which had been in the red for a long time until Q3 last year, has been in the black since Q4 last year. This is due to the strong performance of its epilepsy drug Xcopri (cenobamate) in the U.S. market. Xcopir’s
- Company
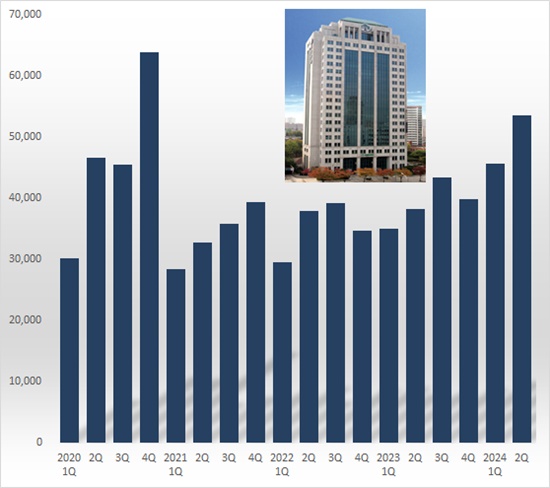
- Celltrion achieved highest ever sales in Q2
- by Hwang, Byung-woo Aug 8, 2024 03:37pm
- Celltrion has topped quarterly sales of KRW 800 billion the first in the company's history, driven by the successful sales of newly launched biosimilar product. According to Celltrion's consolidated income statement on August 7th, its sales this year amounted to KRW 874.7 billion, up 66.9% year over year (YOY). This figure is a record high in
- Company
- Revisions to the guidelines for atopic dermatitis treatment
- by Hwang, Byung-woo Aug 8, 2024 09:25am
- As the market for atopic dermatitis rapidly shifts due to the introduction of new drugs, domestic guidelines are being revised in nine years, and therapeutic guidelines are also changing. The guidelines now suggest a higher recommendation grade for treatments, including biological agents and JAK inhibitors, for use in patients with moderate-t
- Company
- RSV vaccine market for children is expected to be big
- by Hwang, Byung-woo Aug 8, 2024 09:24am
- As Sanofi is set to launch its injectable antibody drug to prevent respiratory syncytial virus (RSV) lower respiratory tract disease for the first time in South Korea, the company begins marketing. The company aims to raise awareness of RSV disease to promote vaccination. Sanofi has strategized a top-down approach, starting with labor and de
- Company
- Celltrion presents clinical results of Prolia biosimilar
- by Hwang, Byung-woo Aug 8, 2024 09:24am
- Celltrion announced on August 6th that it has published the global Phase 3 clinical trial results evaluating the efficacy and safety of CT-P41, a biosimilar referencing the osteoporosis drug Prolia (ingredient: denosumab). The Phase 3 study compared the safety profile, including the efficacy, pharmacodynamics, pharmacokinetics, and immuno
- Company
- Qalsody receives orphan drug designation in Korea
- by Eo, Yun-Ho Aug 8, 2024 09:24am
- ‘Qalsody (tofersen),’ a new drug for Lou Gehrig's disease, was designated as an orphan drug in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the designation through an orphan drug designation notice. More specifically, the drug is indicated for amyotrophic lateral sclerosis (Lou Gehrig's disease) associated wi
- Company
- Vaxneuvance increases presence in NIP mkt
- by Hwang, Byung-woo Aug 8, 2024 09:24am
- MSD Korea is accelerating its efforts to capture the national immunization program (NIP) market by touting the high immunogenicity of Vaxneuvance. The vaccine has already been rapidly introduced to general hospitals and clinics upon its launch, and the company is highlighting the vaccine’s clinical benefits to gain a competitive advantage.
- Company
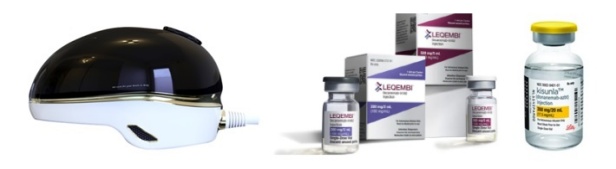
- Electronic drugs show potential to conquering Alzheimer's
- by Son, Hyung-Min Aug 8, 2024 09:24am
- The Korean pharmaceutical bio industry is making a bid into the electronic drug market for Alzheimer's disease. Recently, Remed unveiled the results of its transcranial magnetic stimulation (TMS) therapy that demonstrated an effect in treating Alzheimer's disease. AriBio, which is developing a new drug for Alzheimer's disease, is developing an e