- Company
- Tepezza receives orphan drug designation in Korea
- by Eo, Yun-Ho Aug 6, 2024 09:16am
- Tepezza (teprotumumab), a targeted treatment for thyroid eye disease (TED), has been designated as an orphan drug in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the designation through an orphan drug designation notification. Specifically, Tepezza is indicated for the treatment of adult patients with moderate-
- Company
- Lily Korea appoints John Bickel as new General Manager
- by Hwang, Byung-woo Aug 6, 2024 09:16am
- Eli Lilly Korea announced on August 5th the appointment of John Bickel as the new General Manager of Eli Lilly Korea, effective August 1st. Bickel is an expert with 26 years of extensive leadership experience at Eli Lilly and Company. In the headquarters, he was responsible for the U.S. and global market in oncology and neuroscience divis
- Company
- Trials start for 'cancer vaccine+immunooncology drug’ combo
- by Son, Hyung-Min Aug 6, 2024 09:16am
- Global pharmaceutical companies are making progress in developing messenger ribonucleic acid (mRNA) cancer vaccines and immuno-oncology combinations. Recently, Regeneron and BioNTech confirmed efficacy in a Phase II clinical trial for melanoma. Moderna, a company specializing in the development of mRNA vaccines, has also shown promise in mela
- Company
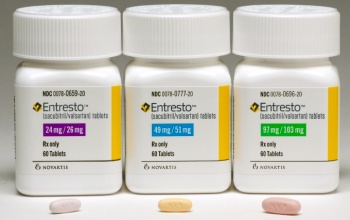
- Entresto sales soar… rises 22% in 1 year
- by Kim, Jin-Gu Aug 6, 2024 09:15am
- Sales of Novartis' heart failure drug 'Entresto' continues to grow over 20% YoY in its 7th year on the market. At this rate, the drug is expected to generate prescription sales of around KRW 70 billion by the end of this year. The variable is the patent dispute. The company is currently awaiting its second judgment on its patent dispute w
- Company
- Nabota records KRW 92B in North American sales in Q2
- by Son, Hyung-Min Aug 5, 2024 07:51am
- Evolus has posted record quarterly sales thanks to a surge in sales of its botulinum toxin Nabota. The company generated USD 126 million in sales in the first half of the year, lighting a green light to achieving its performance target for the year. Evolus plans to continue to grow sales by expanding Nabota's international approvals and launchin
- Company
- Pemazyre may be prescribed at general hospitals in Korea
- by Eo, Yun-Ho Aug 5, 2024 07:51am
- Pemazyre, a treatment for intrahepatic bile duct cancer(cholangiocarcinoma) can now be prescribed at general hospitals in Korea. According to industry sources, Handok's Pemazyre (pemigatinib) has passed the drug committee (DC) of top general hospitals in Korea including Samsung Medical Center, Seoul National University Hospital, and Asan
- Company
- Obesity drugs, 'Wegovy vs Mounjaro' after expanded approval
- by Hwang, Byung-woo Aug 5, 2024 07:51am
- Wegovy and Mounjaro, regarded as next-generation treatments for obesity, have expanded indications. As a result, intense competition in the market is expected. On July 1st, Eli Lily's Mounjaro (ingredient: tirzepatide) received approval as an adjunct for chronic weight management, a year after it received approval as a type 2 diabetes tre
- Company
- Pulmican·Pulmicort prescriptions surge in asthma Tx mkt
- by Chon, Seung-Hyun Aug 5, 2024 07:50am
- The prescription market for 'budesonide' asthma drugs has expanded significantly. The drug price hike at the end of last year led to increased production and an expansion of the prescription market. The prescription market has also shown growth due to the resolution of the supply and demand imbalance made for drugs with supply and demand imbalan
- Company
- Bosulif, 2nd-generation leukemia drug available in Big 5
- by Eo, Yun-Ho Aug 5, 2024 07:50am
- The second-generation leukemia drug, 'Bosulif,' has landed in 'Big 5' general hospitals. According to industry sources, Pfizer Korea's chronic myelogenous leukemia (CML) drug, Bosulif, has passed the drug committees (DC) of tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's
- Company
- Fresenius Kabi launches 2 Ntense amino acid solutions
- by Eo, Yun-Ho Aug 2, 2024 05:52am
- On the 1st, Fresenius Kabi Korea (CEO&President: Noah Park) announced the launch of its high-strength amino acid nutritional solutions ‘Ntense’ and ‘Ntense EF’ in Korea. Ntense, which stands for “Nitrogen Intensify,” is a three-chamber bag product that provides ▲nutritional treatment for critically ill patients with a high risk of