- Company
- Botulinum toxin exports in 1H rise 17%
- by Kim, Jin-Gu Jul 17, 2024 05:50am
- Korea's botulinum toxin exports increased by 17% year-on-year in the first half of this year. This is the highest half-yearly export performance recorded ever. The increase in exports to the United States, China, and Japan is analyzed to have driven the increase in overall botulinum toxin exports. According to the Korea Customs Service on Jul
- Company
- Ultomiris gets expanded indication for neuromyelitis optica
- by Hwang, Byung-woo Jul 17, 2024 05:50am
- Ultomiris (ravulizumab) has strengthened its position in the market for neuromyelitis optica after obtaining approval for expanded indication. On July 11th, the Ministry of Food and Drug Safety (MFDS) granted approval of expanded indication for Ultomiris, a C5 complement inhibitor, for the treatment of adults aged 18 years and above with n
- Company
- Will Eylea K-biosimilar overcome evergreening in the U.S.?
- by Hwang, Byung-woo Jul 16, 2024 05:48am
- The expiration of the U.S. substance patent for the blockbuster biopharmaceutical Eylea (aflibercept) is expected to spark competition in the biosimilar market. Samsung Bioepis, which preemptively received marketing authorization for an Eylea biosimilar, is stuck in a patent dispute with Regeneron, which owns the original Eylea. It is expecte
- Company
- Bladder cancer drug Balversa lands in KOR 1.5yr after apvl
- by Eo, Yun-Ho Jul 15, 2024 05:47am
- The new bladder cancer drug Balversa may now be prescribed, one year and a half after being approved in Korea. According to industry sources, Janssen Korea’s FGFR targeting urothelial carcinoma (bladder cancer) drug Balversa (erdafitinib) recently passed Seoul Asan Medical Center’s drug committee (DC) review. Balversa was approved b
- Company
- Severe asthma drug 'Fasenra' lands in general hospitals
- by Eo, Yun-Ho Jul 15, 2024 05:47am
- ‘Fasenra,’ a severe asthma treatment, has been listed for insurance reimbursement this month and is now available for prescriptions at general hospitals. Industry sources said that AstraZeneca Korea’s Fasenra (benralizumab) has passed the drug committees (DC) of tertiary general hospitals, including Samsung Medical Center in Seoul, Se
- Company
- K-Bios make progress in new rare lung cancer drug dev
- by Son, Hyung-Min Jul 15, 2024 05:47am
- The c-MET-mutation-targeted NSCLC drugs that are being developed by Korean pharma and biotech companies have been recognized for their potential. Recently, Abion Bio signed an agreement with Janssen to receive Leclaza (lasertinib) free of charge. This agreement will allow Abion Bio to commercialize the combination of its in-development vabamet
- Company
- K-Bio speeds up novel drug development to treat MASH
- by Son, Hyung-Min Jul 15, 2024 05:47am
- The biotech industry in South Korea has made notable achievements in treating MASH. Many pharmaceutical companies have failed in clinical trials to develop a treatment for MASH due to its complex pathogenesis. As the first treatment for MASH has been approved, the industry draws attention to the success of the commercialization of other new drug
- Company
- Protein C deficiency drug 'Ceprotin' begins negotiations
- by Eo, Yun-Ho Jul 12, 2024 05:48am
- 'Ceprotin' has entered the last stage of obtaininig reimbursement listing. According to our investigation, Takeda Pharmaceuticals Korea has started to negotiate drug pricing with the National Health Insurance Service (NHIS) for Ceprotin (Human potein C), a novel drug for the treatment of congenital protein C deficiency. ‘Human protei
- Company
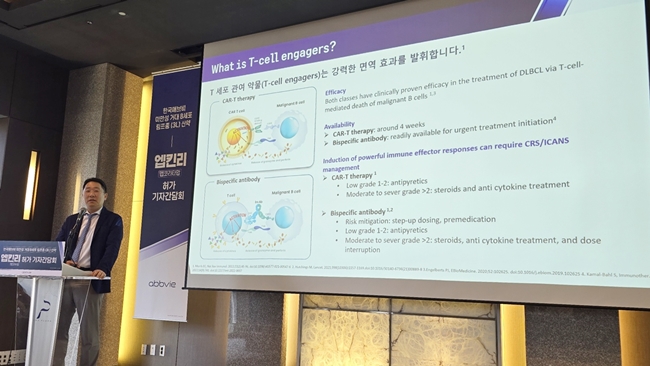
- Will Epkinly become a game-changer in the blood cancer mkt?
- by Hwang, Byung-woo Jul 12, 2024 05:47am
- The arrival of bispecific antibody-based therapies in Korea is expected to shift the blood cancer treatment market paradigm. The industry is welcoming the introduction of a new treatment option, as an unmet need exists in diffuse large B-cell lymphoma (DLBCL), which has a poor prognosis even after three or later lines of treatments. Ab
- Company
- "Enhertu gets expanded indication to treat lung cancer"
- by Son, Hyung-Min Jul 11, 2024 06:12am
- “Previously, patients could only use Enhertu by participating in clinical trials conducted in tertiary general hospitals. Current approval in South Korea will provide a new treatment option for patients with HER2 mutant metastatic non-small cell lung cancer (NSCLC). Brain metastasis is common in lung cancer patients. Because Enhertu demonst