- Company
- ERT demonstrates effectiveness in treating Fabry disease
- by Whang, byung-woo Oct 11, 2024 06:25am
- "'It has been 20 years since ERT treatments became available for patients with Fabry disease. More treatment options are available to treat Fabry disease, but selecting treatment options and strategy requires precise attention based on research data." Analysis suggests that the treatment environment for rare diseases has improved due to the e
- Company
- ‘Switching between AD drugs’ discussed at NA audit
- by Whang, byung-woo Oct 11, 2024 05:54am
- The issue of switching between medications for severe atopic dermatitis, whose need had been voiced constantly in the clinical field, has been mentioned during the National Assembly Audit, attracting attention to whether the reimbursement environment will change. During the National Audit of the National Assembly's Health and Welfare Co
- Policy
- MFDS Minister ‘will work with MOHW on INN prescriptions’
- by Lee, Hye-Kyung Oct 11, 2024 05:54am
- International non-proprietary names (INNs) and ingredient-based prescriptions should be introduced to prevent patients from circling pharmacies due to drug shortages, said a lawmaker. ‘Institutional inducements such as the introduction of INNs and ingredient-name prescriptions are needed to prevent drug supply disruptions and pharmacy bounci
- Opinion
- [Reporter's view] Creating a virtuous cycle for K-bio growth
- by Son, Hyung Min Oct 11, 2024 05:54am
- The research and development (R&D) trend in the biopharmaceutical industry is focusing on new anticancer drugs. The field is gaining interest, especially following the clinical success of Yuhan Corp's Leclaza (ingredient: Lazertinib) and HLB's rivoceranib. In particular, most biotech companies in South Korea are confirming the clinical po
- Company
- Will Tevimbra be reimbursed promptly in Korea?
- by Eo, Yun-Ho Oct 11, 2024 05:54am
- Whether an immuno-oncology treatment option will arise in the oesophageal cancer space is garnering attention. BeiGene Korea's immuno-oncology drug Tevimbra (tislelizumab) passed the Health Insurance Review and Assessment Service’s Cancer Disease Review Committee in August during its second attempt. Tevimbra is a PD-1 inhibiting immuno-o
- Company
- SK bioscience invests KRW 4.1B stake in a U.S. biotech
- by Kim, Jin-Gu Oct 10, 2024 05:51am
- SK bioscience announced on October 8th that the company has signed an agreement to acquire a stake in U.S. biotech Fina Biosolutions by investing USD 3 million (approximately KRW 4.1 billion). SK bioscience became the first and only strategic investor of Fina Biosolutions. According to the contract between both companies, the specific stake
- Company
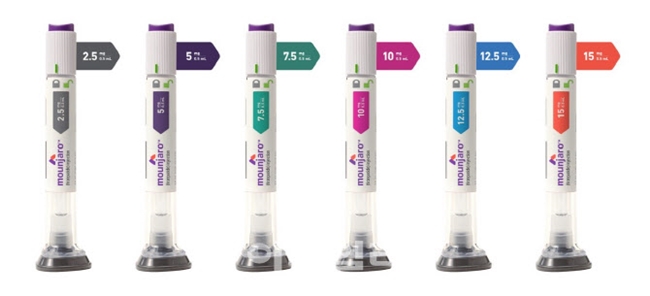
- Who will distribute Mounjaro in Korea?
- by Whang, byung-woo Oct 10, 2024 05:50am
- As Novo Nordisk's obesity drug Wegovy (semaglutide) is set to be released, the industry’s interest is on which domestic distributor Lilly will choose for its Mounjaro (tirzepatide). According to industry sources on the 8th, Boryung Pharmaceutical and Chong Kun Dang are in the running to become domestic distributors of Wegovy’s rival ob
- Policy
- "Preferential drug pricing excludes K-made new drug benefits
- by Lee, Jeong-Hwan Oct 10, 2024 05:50am
- The government's revised plan for the drug pricing system has been criticized for reverse discrimination against Korean pharmaceutical companies because it was primarily designed to favor multinational pharmaceutical companies. The criticism concerns the revised drug pricing system plan, announced by the Health Insurance Review and Assessment
- Opinion
- [Reporter's View]Industry expectations rise with new changes
- by Eo, Yun-Ho Oct 10, 2024 05:50am
- The criteria for innovative new drugs that are eligible for the flexible application of the ICER threshold and the measures for the expansion of the risk-sharing agreement (RSA) have been revealed. As always, the industry expressed a mix of expectations and concerns. The disclosed ‘Detailed evaluation criteria for new drugs etc. subject
- Policy
- Yoon Kim ‘40% of the generic drug price is a bubble’
- by Lee, Jeong-Hwan Oct 10, 2024 05:50am
- Kyoo-hong Cho, the Minister of Health and Welfare, positively responded to Democratic Party of Korea Representative Yoon Kim's request to lower the price of generic versions of patent-expired drugs within the year. This refers to a separate generic drug price cut rather than the foreign drug price referencing reevaluations, which the phar