- Company
- New drug Voranigo receives orphan drug designation in Korea
- by Eo, Yun-Ho Sep 4, 2024 05:49am
- Voranigo, the first new malignant brain tumor drug introduced in 20 years, has received an orphan drug designation in Korea. The Ministry of Food and Drug Safety announced so through an official orphan drug designation notice on the 3rd. Specifically, the drug is for the treatment of IDH-mutated diffuse glioma. Voranigo can be used to
- Company
- Yuhan's investment in US subsidiary total KRW 27B over 5 yrs
- by Chon, Seung-Hyun Sep 4, 2024 05:48am
- Yuhan Corporation has invested KRW 3.9 billion additionally to the U.S.-based subsidiary of the company. The company invested KRW 27 billion over the past five years, expanding support for global entry. According to Financial Supervisory Service on September 4th, Yuhan Corporation invested KRW 3.9 billion in the first half of the year and acq
- Company
- Generic companies lastly challenge Lixiana patent
- by Kim, Jin-Gu Sep 4, 2024 05:48am
- Generic companies have been filing late patent challenges against Lixiana (edoxaban), the market leader in the direct-acting oral anticoagulants (DOACs) market. This is due to the imminent expiry of the product's formulation patent, which is 2 years away. The strategy of the patent challengers is to avoid the formulation patent and laun
- Policy
- Alvogen voluntarily recalls Comtan Tab due to impurities
- by Lee, Hye-Kyung Sep 3, 2024 05:53am
- Single-agent entacapones, which are used for Parkinson's syndrome, are being recalled due to the detection of excess impurities. The issue of drug impurities continues to arise after MFDS has added nitrosamine impurity testing as a quality control test item to strengthen the management of nitrosamine impurities. On March 30, MFDS ann
- Company
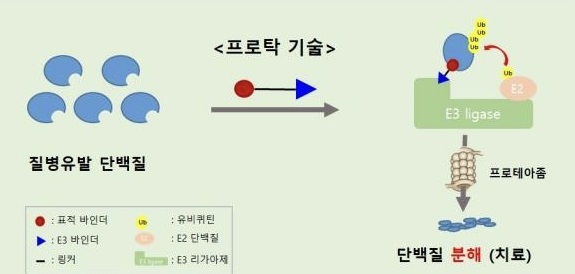
- Pharma companies jump into developing 'TPD' for cancer
- by Son, Hyung-Min Sep 3, 2024 05:53am
- The global and Korean pharmaceutical industries are focusing on securing Targeted Protein Degradation (TPD) technology, which has emerged following the success of Antibody-Drug Conjugates (ADCs). Recently, major global pharmaceutical companies such as Pfizer, Novartis, and Eli Lilly have successfully acquired TPD technologies, marking TPD as a n
- Policy
- Prolia’s drug price cut 30% before the entry of biosimilars
- by Lee, Tak-Sun Sep 3, 2024 05:53am
- Prolia, the No. 1 product in the domestic osteoporosis treatment market, has had its upper insurance price limit adjusted this month through price-volume agreement (PVA) negotiations. The drug underwent 4 PVA negotiations in total, which led to a price drop of nearly 30% from the initial price it received in October 2017 upon reimbursement.
- Policy
- Rival party heads discuss pending issue in NA
- by Lee, Jeong-Hwan Sep 3, 2024 05:53am
- Dong-hoon Han, Chairman of the People Power Party, and Jae-Myung Lee, Chairman of the Democratic Party of Korea met at the National Assembly on the afternoon of the 1st and reached a consensus on resolving the parliamentary conflicts and medical gap. Han mentioned the need to resolve the public's anxiety over the medical gap, while Lee sugge
- Policy
- Usage recommendations for 3 JAK inhibitors to be changed
- by Lee, Tak-Sun Sep 3, 2024 05:53am
- The Ministry of Food and Drug Safety (MFDS) will implement changes to approval for Janus Kinase (JAK) inhibitors, recommending low dosages when used to treat high-risk patients. This is a follow-up measure to the changes to the efficacy and effectiveness section for high-risk patient treatment in 2022. On August 30th, the MFDS posted
- Policy
- Pfizer's new COVID-19 vaccine wins nod in KOR
- by Lee, Hye-Kyung Sep 2, 2024 05:48am
- The new vaccine, JN.1, developed against emerging COVID-19 subvariant, received approval in South Korea. The Ministry of Food and Drug Safety (MFDS) announced on August 30th that it had completed approval just under two and a half months after application was submitted. A designated review team was formed following the plan announced by t
- Company
- Biological drug options for psoriasis treatment increase
- by Son, Hyung-Min Sep 2, 2024 05:48am
- Domestic and foreign pharmaceutical companies are expanding psoriasis treatment options through the development of biological agents. Recently, Korea's UCB Pharma succeeded in obtaining domestic marketing authorization for a biological drug with a new mechanism of action, sparking competition in the field. Other domestic pharmaceutical and biote