- Company
- Korean pharma and biotechs expand R&D investments
- by Chon, Seung-Hyun Aug 29, 2024 04:32am
- Listed pharma and biotech companies have expanded their research and development (R&D) investments in Korea this year. 3 out of 5 major pharmaceutical companies increased their R&D investments from last year to discover new candidates. Pharmaceutical companies with larger sales have been more active in reinvesting in R&D. According to the Fin
- Company
- Breast cancer drug Trodelvy receives DREC deliberations
- by Eo, Yun-Ho Aug 29, 2024 04:31am
- The ADC breast cancer drug Trodelvy has made a step toward insurance reimbursement after 9 months of wait. Gilead Sciences' triple-negative breast cancer (TNBC) drug Troldelvy, whose reimbursement request received 100,000 consents in a public petition, will be presented for deliberation to the Health Insurance Review and Assessment Servic
- Policy
- Will RWD-based reimb agreements be more beneficial?
- by Lee, Tak-Sun Aug 29, 2024 04:31am
- Drug reimbursement contracts satisfy all parties involved when they achieve three goals: The first is patient access, the second is sustained revenue for the pharmaceutical company, and the third is budget management for the payer. “A fair price is one that can satisfy both the seller and the buyer,” said So-young Lee, Director of Hea
- Opinion
- [Reporter’s View]Support domestic COVID-19 drugs
- by Lee, Hye-Kyung Aug 28, 2024 05:52am
- The recurrence of COVID-19 has sparked interest in COVID-19 drugs. After the pandemic turned into an endemic, the government set a budget of KRW 179.8 billion for COVID-19 drugs this year, which was a 53.2% cut from last year, and the number of COVID-19 drugs introduced in Q1 and Q2 of this year was 179,000, half of the 341,000 that the gove
- Company
- "Switching of atopic dermatitis treatments not yet allowed"
- by Hwang, Byung-woo Aug 28, 2024 05:52am
- As new drug entries shift the market for atopic dermatitis, the public onion demands changes to reimbursement assessment, such as considering the comprehensive factors to use increased treatment options efficiently. Opinions have been suggested to consider factors related to patient quality of life, such as improving itchiness, in addition to
- Policy
- Price of 17 items cut 30% during Type C PVA negotiations
- by Lee, Tak-Sun Aug 28, 2024 05:52am
- The National Health Insurance Service (NHIS) has announced that it has achieved health insurance financial savings of KRW 52.1 billion (USD 52.1 million) through ‘Type C’ Price-Volume Agreement (PVA) negotiations this year. This is an 85.5% increase from the KRW 28.1 billion in the previous year. In particular, the revision of the deta
- Company
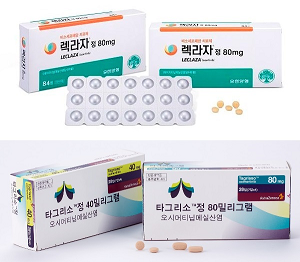
- K-made Leclaza monotherapy to be showcased at global meeting
- by Son, Hyung-Min Aug 28, 2024 05:52am
- Korean biopharmaceutical companies continue to gain R&D achievements for targeted anti-cancer drugs. Companies have prepared to present their Korean-made anti-cancer drugs, including Leclaza and Rivoceranib at the 2024 World Conference on Lung Cancer, which will be held September 7-10 in San Diego, USA. Yuhan Pharmaceutical will present
- Policy
- MFDS to review side effects relief imposed on companies
- by Lee, Jeong-Hwan Aug 28, 2024 05:52am
- The Ministry of Food and Drug Safety (MFDS) announced that it would try to set the reasonably sized 'Payment for Benefits for relief of Injury from Side Effects of Drugs, (hereafter referred to as payment for benefits for relief) ' which is currently charged to pharmaceutical companies. MFDS' audit of the Korea Institute of Drug Safety
- Company
- Challenges in the evolving lung cancer treatment landscape
- by Son, Hyung-Min Aug 28, 2024 05:51am
- The head-to-head battle between the epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer drugs (NSCLC) Leclaza and Tagrisso has carried on to competition of each drug's combination therapy regimens. This month, the U.S. Food and Drug Administration (FDA) approved Leclaza+Rybrevant as a first-line treatment for EGFR-positive
- Company
- Value of new drugs should be evaluated based on efficacy
- by Hwang, Byung-woo Aug 27, 2024 05:50am
- 'Receiving recognization of the value of new drugs' is one of the key challenges for pharma and biotech companies. With the proportion of new drugs being introduced into Korea rising and the number of homegrown new drugs increasing, the need for a drug pricing policy that recognizes the value of these new drugs has been growing. Recog