- Policy
- Samjin and Korea Pharma’s first generics are reimb in KOR
- by Lee, Tak-Sun Jul 25, 2024 05:51am
- Samjin Pharmaceutical and Korea Pharma have launched first generics in Korea. Samjin Pharmaceutical will launch the first generic of Sanofi's atrial fibrillation drug Multaq Tab, while Korea Pharma will launch the first generic of Pfizer's antidepressant Pristiq ER Tab. According to industry sources, Samjin Pharmaceutical's ‘Samjin Dron
- Company
- Meningitis B vaccine Bexsero is released in Korea
- by Moon, sung-ho Jul 25, 2024 05:51am
- Competition in the 'meningococcal' vaccine market, which is mainly vaccinated in pediatric clinics, has recently been reignited. Although its domestic market is worth less than KRW 10 billion, the emergence of next-generation vaccines is expected to spark new competition among multinational pharmaceutical companies. This is because GSK
- Company
- 'Padcev+Keytruda' combination therapy is set to land in KOR
- by Eo, Yun-Ho Jul 25, 2024 05:51am
- The combination therapy of 'Padcev+Keytruda,' which is expected to bring a paradigm shift to bladder cancer treatment, will soon land in South Korea. The Ministry of Food and Drug Safety (MFDS) is reviewing the expansion of indication for Astellas Korea's Padcev (enfortumab), an antibody-drug conjugate (ADC), in combination with Keytruda
- Opinion
- [Reporter's View] Keeping up with the Chinese bio industry
- by Son, Hyung-Min Jul 24, 2024 05:51am
- The research and development (R&D) capabilities of Chinese pharmaceutical companies are going from strength to strength year after year. Last year, the immuno-oncology drug Loqtorzi, developed by Junshi Biosciences, was approved in the United States. It became the first Chinese immuno-oncology drug to be approved by the U.S. Food and Dru
- Policy
- Gvt commences 'Machine Learning AI Project' for new drug dev
- by Lee, Jeong-Hwan Jul 24, 2024 05:51am
- The government will actively pursue a project for faster new drug discovery. It will fund KRW 34.8 billion over 5 years, from 2024 to 2028, and conduct a project utilizing federated learning of new drug discovery data owned by pharmaceutical companies, university hospitals, research institutes, and corporations. The Ministry of Health
- Policy
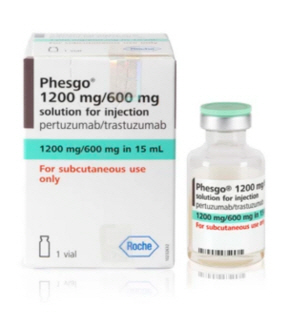
- Roche’s Phesgo is applied RSA for reimb in Korea
- by Lee, Tak-Sun Jul 24, 2024 05:51am
- Korea’s insurance authorities are introducing various measures to reduce drug costs. The government is applying risk-sharing agreements to precalculation drugs and requiring submission of follow-up data for drugs that are exempt from sumitting pharmacoeconomic evaluation data. The drugs subject to the authorities’ measures are Phesgo SC
- Company
- Samsung Bioepis’ Soliris biosimilar is approved in the U.S.
- by Chon, Seung-Hyun Jul 24, 2024 05:51am
- [데일리팜=천승현 기자] 삼성바이오에피스는 미국 식품의약품국(FDA)으로부터 희귀질환치료제 ‘에피스클리’의 품목허가를 획득했다고 23일 밝혔다. 에피스클리는 미국 알렉시온이 개발한 솔리리스의 바이오시밀러 제품이다. 에피스클리는 발작성 야간 혈색소뇨증, 비정형 용혈성 요독 증후군의 치료제로 FDA 승인을 받았다. 삼성바이오에피스는 2019년 7월부
- Company
- Reimb applied for new HIV drug 'Vocabria+Rekambys'
- by Eo, Yun-Ho Jul 24, 2024 05:50am
- Long-acting HIV treatment 'Vocabria+Rekambys' combination therapy aims to be listed for insurance reimbursement after receiving approval in South Korea two years ago. Industry sources said that GSK Korea and Janssen Korea have applied for reimbursement for the combination therapy of Vocabria (cabotegravir) and Rekambys (rilpivirine), whic
- Company
- Will 'Zejula' be reimbursed for HRD-positive ovarian cancer?
- by Eo, Yun-Ho Jul 23, 2024 05:48am
- Whether the insurance reimbursement criteria for 'Zejula,' a PARP inhibitor, will include 'HRD-positive' is gaining attention. Sources said that Takeda Pharmaceuticals Korea is negotiating the price with the National Health Insurance Service (NHIS) for the Poly ADP-ribose Polymerase (PARP) inhibitor, Zejula, which is used to treat ovarian
- Company
- Opdivo approved as 1st-line Tx for urothelial cell carcinoma
- by Hwang, Byung-woo Jul 23, 2024 05:48am
- Ono Pharma Korea and BMS Korea announced on the 22nd that Opdivo (nivolumab) has been additionally approved by the Ministry of Food and Drug Safety as a first-line treatment for urothelial cell carcinoma (UCC). The new indication is for the first-line treatment of unresectable or metastatic urothelial cell carcinoma in combination with ci