- Company
- 'Increased' competitiveness in autoimmune diseases
- by Son, Hyung-Min Jun 27, 2024 05:47am
- The Korean biopharmaceutical industry is successfully signing license agreements of drugs for autoimmune diseases. HK inno. N, IMBiologics, and Y-Biologics have signed license agreements for their jointly developed novel drug candidates. AprilBio has also signed a license agreement for a novel drug candidate that has shown efficacy in a phase 1
- Policy
- Some Akynzeo products recalled due to insufficient API
- by Lee, Hye-Kyung Jun 27, 2024 05:47am
- The Ministry of Food and Drug Safety (MFDS) recalled some lot numbers of HK Inno.N's antiemetic ‘Akynzeo Cap’ after confirming the possibility that the drug may not contain enough active&160;pharmaceutical ingredient (API). On the 21st, the MFDS issued a recall order for Akynzeo batch number '43000563 [2026-11-30].’ The reason fo
- Company
- New drug 'Mylotarg' for AML makes another attempt at reimb
- by Eo, Yun-Ho Jun 27, 2024 05:47am
- A new drug 'Mylotarg' for the treatment of acute myeloid leukemia (AML) is making another attempt to obtain insurance reimbursement listing. According to industry sources, Pfizer Korea has recently reapplied for reimbursement review for Mylotarg (gemtuzumab ozogamicin), a treatment for acute myeloid leukemia (AML). In May 2022, Mylota
- InterView
- ‘Leqembi enables prevention of Alzheimer's disease’
- by Son, Hyung-Min Jun 27, 2024 05:47am
- "Now that there is a treatment for Alzheimer's disease, we can prevent dementia by 90% if it is detected early. With better drugs expected to be developed in the future, we should provide timely treatment for the patients so they can live to grasp that new opportunity." Duk Lyul Na, Director of the Happymind Clinic (former Professor of Ne
- Company
- SGLT-2 diabetes drug Jardiance outsells mkt leader Forxiga
- by Nho, Byung Chul Jun 26, 2024 05:47am
- Boehringer Ingelheim's Jardiance and AstraZeneca's Forxiga are striving for mastery of the KRW 110 billion SGLT-2 inhibitor class single-agent diabetes drug market in Korea. In the first quarter of this year, Jardiance and Forxiga generated sales in the range of KRW 11.1 billion and KRW 8.0 billion, respectively. Notably, this is a reve
- Boryung’s early release of its Lenvima generic unclear
- by Kim, Jin-Gu Jun 26, 2024 05:46am
- Boryung’s plan to launch a generic version of the liver cancer drug Lenvima (lenvatinib) early to coincide with the expiration of Lenvima’s product patent is facing setbacks. Despite the amount of time spent overcoming the patents, the number of remaining patents seems to be increasing rather than decreasing. With less than a year left
- Company
- Another anticancer drug 'Augtyro' set to be launched in KOR
- by Eo, Yun-Ho Jun 26, 2024 05:46am
- A ROS-1 targeting lung cancer treatment called 'Augtyro' is entering the Korean market. According to the industry sources, Bristol Myers Squibb (BMS) Korea has recently applied for market approval from the Ministry of Food and Drug Safety (MFDS) for its Augtyro (repotrectinib), an anticancer drug effective regardless of cancer types. T
- Policy
- ‘Hypertension·hyperlipidemia generics are pricier in KOR'
- by Lee, Jeong-Hwan Jun 26, 2024 05:46am
- According to a government study, generic drugs for some indications, such as those for the gastrointestinal system, hypertension, and hyperlipidemia, are more expensive in Korea than in major overseas countries other than the United States, such as the United Kingdom, Switzerland, and Japan. As of 2022, Korean generic hyperlipidemia drugs we
- Company
- Returned license-outs for new drugs one after another
- by Kim, Jin-Gu Jun 26, 2024 05:46am
- Korean biopharmaceutical companies have been receiving termination notifications from their partnering companies for their licensed-out new drug candidates one after another. Olix Pharmaceuticals and Curacle recently received notifications of contract terminations from France’s Thea Open Innovation. Voronoi’s license-out agreement for
- Policy
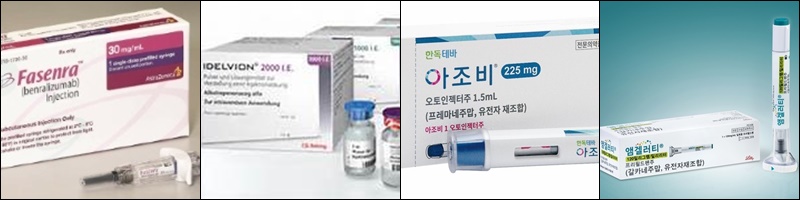
- Fasenra·Idelvion, Ajovy·Emgality receive reimb in KOR
- by Lee, Jeong-Hwan Jun 25, 2024 05:47am
- AstraZeneca's severe eosinophilic asthma treatment Fasenra (benralizumab) and CSL Behring's hemophilia B drug Idelvion (albutrepenonacog alfa) will be reimbursed by the national health insurance starting on the 1st of next month. Also, the anti-malignant tumor agent rituximab (original brand name: MabThera) and the migraine drug Ajovy (f