- Policy
- Approval for PH-ILD inhalation drug 'Tyvaso' expected soon
- by Lee, Hye-Kyung Jun 11, 2024 05:48am
- The approval of 'Tyvaso Inhalation Solution 0.6 mg/mL (treprostinil)' in South Korea is expected soon. It is approved in the United States as the treatment for pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD). According to industry sources on the 11th, the safety and eff
- Company
- GC's Sanfilippo syndrome drug receives fast-track status
- by Son, Hyung-Min Jun 11, 2024 05:47am
- GC Biopharma announced on Tuesday that the U.S. FDA has granted Fast Track Designation for GC1130A, a treatment for Sanfilippo syndrome type A (MPS IIIA) that it has been co-developing with Novel Pharma. The fast-track designation follows the FDA's clearance of the Phase I investigational new drug (IND) application for GC1130A last month
- Policy
- Handok paces its efforts to reimburse Pivlaz in KOR
- by Lee, Tak-Sun Jun 11, 2024 05:47am
- Handok is taking a break from its pursuit of reimbursement for ‘Pivlaz,’ a new drug used to prevent cerebral vasospasm in patients with subarachnoid hemorrhage that the company is supplying and distributing in Korea. The company immediately applied for Pivlaz’s reimbursement to the Health Insurance Review and Assessment Service after
- Company
- 'Novel drugs·CDMO competitiveness↑'
- by Son, Hyung-Min Jun 10, 2024 05:41am
- Major biotech companies in Korea participated in the BIO International Convention (BIO USA 2024) and introduced their in-house competitiveness. Over 50 Korea-based companies attended the BIO USA 2024, held between June 3-6 in San Diego, United States. They sought opportunities to expand partnerships along with discussions for technology tr
- Product
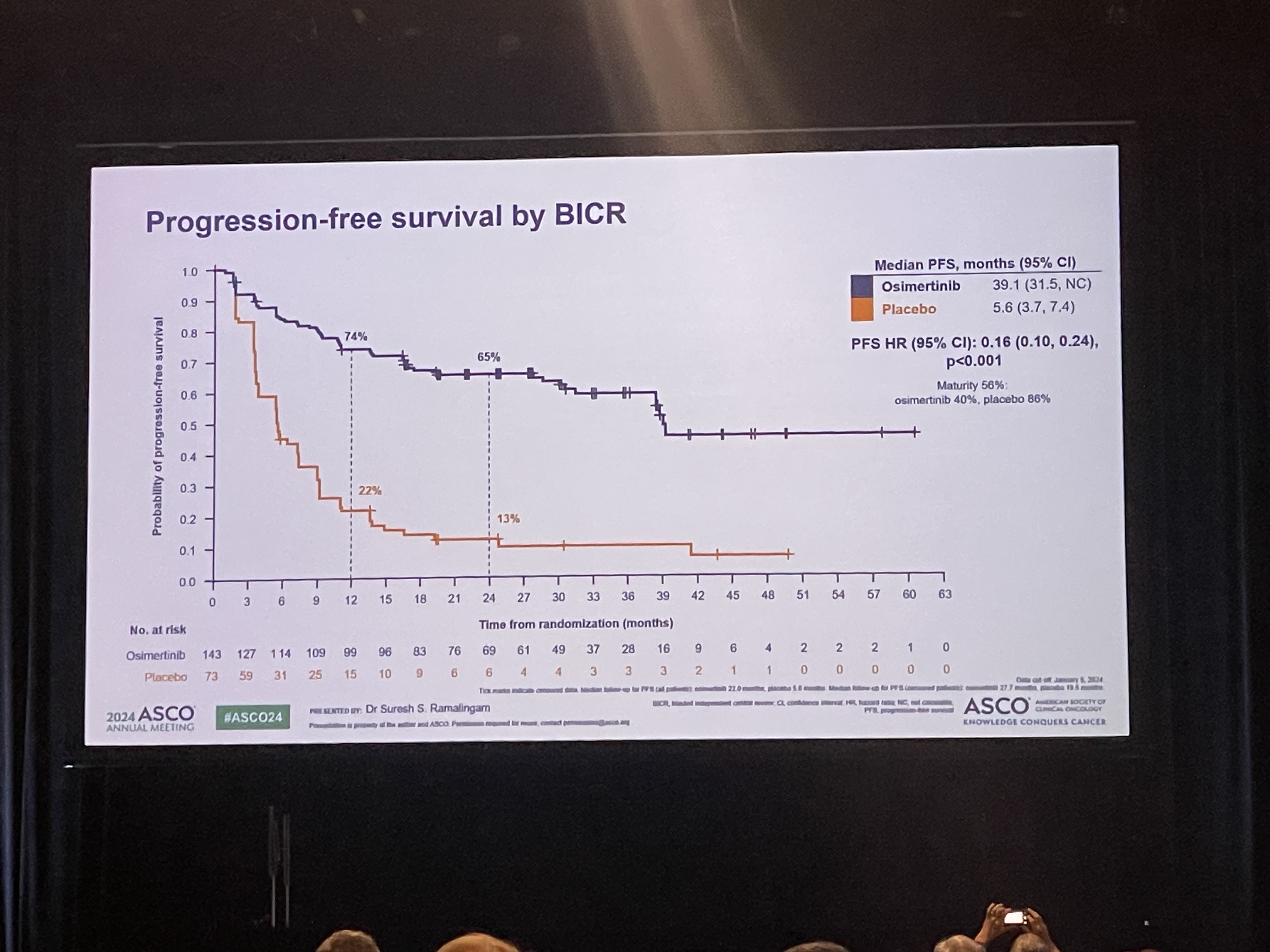
- Will Osimertinib emerge as the standard of care
- by Park, sang-jun Jun 10, 2024 05:41am
- The LAURA trial, which evaluated osimertinib’s effect in patients with unresectable Stage III EGFR-mutant non-small cell lung cancer who received chemoradiotherapy (CRT), was presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. The results were also concurrently published in NEJM. The LAURA trial evaluated
- Company
- Chronic kidney disease drug ‘Kerendia’ can be prescribed
- by Eo, Yun-Ho Jun 10, 2024 05:41am
- ‘Kerendia,’ a treatment for chronic kidney disease, is now available for prescription after receiving approval for insurance reimbursement. According to industry sources, Bayer’s Kerendia (finerenone) has passed the drug committee (DC) of Big 5 tertiary general hospitals, including Seoul National University, Seoul Asan Hospital, and
- Company
- Pharma company MRs illegally work for CSOs on the sideline
- by Lee, Seok-Jun Jun 10, 2024 05:41am
- Contract Sales Organizations (CSOs) are trending in the pharmaceutical industry. In the case of small and medium-sized pharmaceutical companies, the companies have been opting to use CSOs rather than their own sales departments, to the extent that their departments are disappearing with the expansion of CSO business. The smaller the com
- Policy
- Roche applies for Ocrevus' reimbursement in KOR
- by Lee, Tak-Sun Jun 10, 2024 05:41am
- Roche Korea applied for reimbursement of 'Ocrevus Inj. (ocrelizumab, Roche Korea),’ its multiple sclerosis treatment that was approved last month, to the Health Insurance Review and Assessment Service. The drug is administered twice a year, which is considered to have dramatically improved the dosing convenience for MS patients. The d
- Opinion
- [Reporter’s View] Finances limit reimbursement of drugs
- by Eo, Yun-Ho Jun 7, 2024 05:51am
- &160;Insurance reimbursement standards and indications for a drug can differ. This is because the government’s pockets are not infinite under the National Health Insurance System. This is why there are always complaints in the field. Not all complaints can be resolved but there are some that evidently require resolution, that were made
- Company
- Immuno-oncology drugs demonstrate additional benefit at ASCO
- by Son, Hyung-Min Jun 7, 2024 05:50am
- Global pharmaceutical companies have demonstrated their immuno-oncology drugs’ effect in refractory solid tumors. Clinical results from major immuno-oncology drugs, including Imfinzi, Opdivo, and Yervoy, were presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, which kicked off last month in Chicago, U.S.