- Company
- "Breast cancer treatments received positive reviews"
- by Son, Hyung-Min Jun 7, 2024 05:50am
- Major antibody-drug conjugates (ADC) have secured positive clinical results in treating breast cancer. ADCs under development by global pharmaceutical companies have shown to be effective in various types of breast cancer, including triple-negative breast cancer, hormone-positive (HR+)/HER2-negative breast cancer. With these results, latecom
- Company
- ‘Fully utilize the various IBD treatment options available'
- by Eo, Yun-Ho Jun 7, 2024 05:50am
- Incidence of Inflammatory bowel disease (IBD), which had been regarded as a condition typically associated with Westerners, is now rising amongst Asian populations as well, including Koreans. According to the IBD fact sheet published by the Korean Association for the Study of Intestinal Diseases, the number of patients with ulcerative col
- Policy
- Latuda·Dupixent enter negotiations with the NHIS
- by Lee, Tak-Sun Jun 7, 2024 05:50am
- 'Latuda (lurasidone),' a medication used to treat schizophrenia and imported and distributed by Bukwang Pharmaceutical, and 'Dupixent (dupilumab),' which aims to expand reimbursement for young children, have entered negotiations with the National Health Insurance Service (NHIS). When an agreement with the National Health Insurance Service
- Company
- ADC·immunotherapy competitiveness↑…K-bio gains attention
- by Son, Hyung-Min Jun 7, 2024 05:50am
- Korea-based biopharmaceutical companies have confirmed clinical achievements in globally trending R&D areas, including antibody-drug conjugates (ADC), immunotherapy for cancer, and bispecific antibodies. LigaChem Biosciences presented clinical data of LCB14, a human epidermal growth factor receptor 2 (HER2)- targeting ADC candidate. LBC1
- Opinion
- [Reporter's View] Expectations for new Alzheimer's drugs
- by Son, Hyung-Min Jun 5, 2024 05:47am
- Last month, a new drug for Alzheimer's disease, Leqembi, was approved in Korea. Leqembi, which was developed by Eisai and Biogen, targets the amyloid beta (Aβ) protein in the brain, which is considered one of the most likely causes of Alzheimer's disease. The industry welcomed Leqembi’s arrival because there had been no promising new dr
- Opinion
- [Reporter’s View] An open talk on improving the GMP system
- by Lee, Hye-Kyung Jun 5, 2024 05:47am
- Last month, CEOs of biopharmaceutical companies submitted a statement to the Ministry of Food and Drug Safety (MFDS) requesting an improvement to the 'Cancellation of the GMP compliance decision (GMP One strike-out).' They asked that if non-compliance with GMP has been unintentional, a different set of measures be applied instead of one st
- Company
- ‘Hemlibra is the most advanced hemophilia A drug availble'
- by Nho, Byung Chul Jun 5, 2024 05:47am
- "Hemlibra has the potential to become the standard of care for hemophilia A, and is considered the most advanced treatment available." Dr. Midori Shima, professor of pediatric hematology-oncology at Nara Medical University in Japan, said so at a media session to mark the 1st anniversary of Hemlibra's insurance reimbursement extension at
- Company
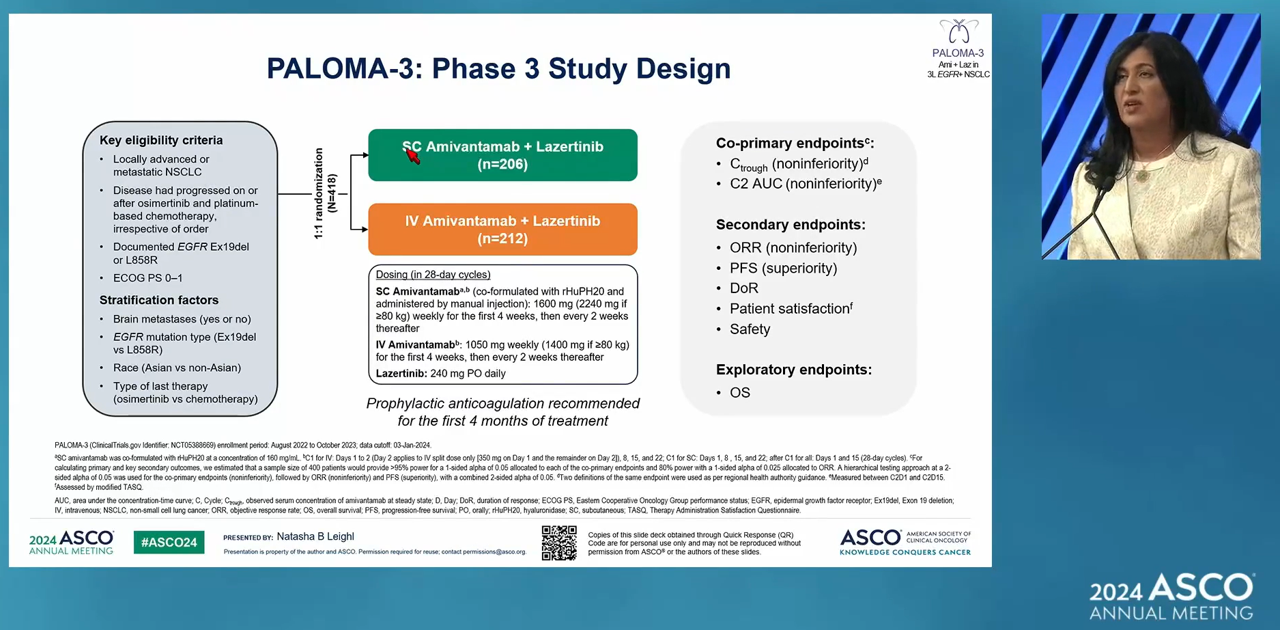
- More data on Leclaza·Lorviqua showcased
- by Son, Hyung-Min Jun 5, 2024 05:47am
- Positive clinical results for targeted therapies, such as EGFR and ALK, were featured at the international conference. Clinical achievements of major targeted therapies for non-small cell lung cancer (NSCLC), including Leclaza, Rybrevant, and Lorviqua, were presented at the American Society of Clinical Oncology (ASCO 2024) annual meeting in Chic
- Policy
- Gov’t will maintain criteria for KIPC recertifications
- by Lee, Jeong-Hwan Jun 5, 2024 05:47am
- The government has decided to maintain the recertification process and criteria for Korea Innovative Pharmaceutical Companies. Therefore, the government decided not to accept the demands of some in the pharmaceutical industry on easing the decertification regulations, ‘2 or more rebate detections and administrative disposition’, and t
- Company
- Sprycel patent to expire soon…market shift worth KRW 40 bil
- by Nho, Byung Chul Jun 4, 2024 05:48am
- Following the patent expiration for blockbuster medicines in the United States this year, generics are expected to enter the market. Likewise, market shifts are expected with the development and launch of generics in South Korea. According to an overseas research agency, the top 10 blockbuster medicines with patents expiring in 2024 inc