- Policy
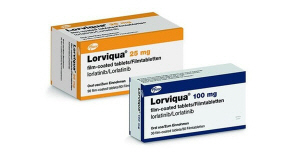
- Lorviqua fails to receive reimb as first-line therapy
- by Lee, Tak-Sun Jun 4, 2024 05:47am
- The reimbursement extension application for Lorviqua, a treatment for anaplastic lymphoma kinase (ALK)-positive non-small-cell cancer, &160;has failed to go beyond the negotiation stage with the National Health Insurance Service. With the breakdown of the negotiations, the company will have to start over from the application stage for Lo
- Company
- Indication for rare cancer drug Retevmo is expanded
- by Son, Hyung-Min Jun 4, 2024 05:47am
- The indication for Retevmo, which targets a rare disease mutation in lung cancer, has been expanded to include children with solid tumors across the spectrum. Retevmo is approved in South Korea for lung cancer but not reimbursed in Korea. It will be interesting to see if the additional confirmation of clinical efficacy will have an impact on
- Company
- Evolution of ADCs… Enhertu prolongs OS in solid tumors
- by Son, Hyung-Min Jun 4, 2024 05:47am
- The antibody-drug conjugate (ADC) anticancer drug Enhertu has demonstrated further efficacy across multiple solid tumors, including HER2-low breast, biliary tract, and head and neck cancers. With the clinical results, the company secured momentum to add more solid tumors to Enhertu’s already established breast, gastric, and non-small cell
- Company
- 'Jemperli,' a PD-1 inhibitor immunotherapy lands in Big 5
- by Eo, Yun-Ho Jun 4, 2024 05:46am
- 'Jemperli,' the first immunotherapy for endometrial cancer, has landed in Big 5 general hospitals. According to industry sources, GSK Korea’s PD-1 inhibitor Jemperli (dostarlimab) has cleared the Drug Committee (DC) of the nationwide medical institutes, including Samsung Medical Center in Seoul, Seoul National University Hospital, Seo
- Policy
- Takeda’s Zejula Tab receives marketing authorization in KOR
- by Lee, Hye-Kyung Jun 3, 2024 05:49am
- Takeda Pharmaceuticals’ poly ADP-ribose polymerase Inhibitor ‘Zejual (niraparib)’ has been granted marketing authorization in Korea in its tablet form. The Ministry of Food and Drug Safety (MFDS) approved Zejula Tab 100 mg on the 31st. Like its capsule formulation, Zejula Tab is indicated ▲as monotherapy for adult patients with ova
- Company
- AbbVie’s Rinvoq is reimbursed for Crohn's Disease in KOR
- by Son, Hyung-Min Jun 3, 2024 05:49am
- Rinvoq’s reimbursement has been extended to cover Crohn's disease and ulcerative colitis in Korea. With this reimbursement extension, Rinvoq became the first and only JAK inhibitor that is reimbursed to treat adults with moderate-to-severe Crohn's disease. The treatment has been shown high effect not only in controlling symptoms but also in
- Company
- 'Dupixent,' the final stages of reimb expansion for children
- by Eo, Yun-Ho Jun 3, 2024 05:48am
- Dupixent is about to enter the last hurdle of expanding insurance reimbursement coverage for young children. According to industry sources, the Ministry of Health and Welfare (MOHW) has recently ordered drug pricing negotiations for Sanofi Korea’s Dupixent (dupilumab). Consequently, a tug-of-war between the government and Sanofi is ab
- Company
- Thrombocytopenia treatment market rises as blue ocean
- by Nho, Byung Chul Jun 3, 2024 05:48am
- The prescription market for idiopathic (immune) thrombocytopenia treatments is expected to grow exponentially with the recent reimbursement standard extension granted in Korea. Until now, reimbursement for oral immune thrombocytopenia drugs has been limited to patients with immune thrombocytopenia who are refractory to corticosteroids a
- Company
- Growing DME market…new drugs clinical trials·biosimilars
- by Son, Hyung-Min May 31, 2024 05:52am
- Korean pharmaceutical companies are challenging the market for diabetic macular edema treatments with oral formulations. Handok and Curacle confirmed effects in clinical trials for oral diabetic macular edema treatments. Because only injectables are available in the market, oral formulations can have a competitive edge because of their convenien
- Company
- Adtralza becomes the 2nd reimbursed biological drug for AD
- by Son, Hyung-Min May 31, 2024 05:52am
- LEO Pharma Korea has launched Adtralza (tralokinumab) as the 2nd biologic drug in the atopic dermatitis market and is on its way to take on Dupixent. LEO Pharma has been emphasizing that Adtralza has a longer dosing interval after 16 weeks compared to existing therapies and is relatively cheaper, reducing the cost burden for patients.