- Company
- "200-day extension for the CMV preemptive therapy Prevymis"
- by Whang, byung-woo Aug 28, 2025 06:10am
- Prevymis (letermovir), a treatment for preventing cytomegalovirus (CMV) infection in allogeneic hematopoietic stem cell transplant patients, is bringing in another paradigm shift as it has entered the '200-day extension criteria.' This drug's influence is expected to grow as the National Health Insurance (NHI) coverage period has been extend
- Company
- Kolon Pharma's active portfolio expansion…new drug·co-dev
- by Son, Hyung Min Aug 28, 2025 06:10am
- Kolon Pharma is increasing its efforts to secure competitiveness in immunology and oncology by combining new drug in-licensing with in-house R&D. Following the introduction of treatments for chronic obstructive pulmonary disease (COPD) and allergies, the company has recently secured a new drug for hypoparathyroidism. Kolon Pharma plans to contin
- Policy
- Bill banning reverse payment agreements passes committee
- by Lee, Jeong-Hwan Aug 28, 2025 06:10am
- The amendment to the National Health Insurance Act, which regulates "reverse payment agreements" where original drug companies and generic drug companies collude by exchanging money to delay or not release generics, thereby avoiding a price reduction for the original drug, passed the National Assembly Health and Welfare Committee review on t
- Opinion
- [Reporter’s View] Addressing the Drug Shortage Issue
- by Kim JiEun Aug 28, 2025 06:09am
- With the bill that revises the Pharmaceutical Affairs Act, centered on simplifying generic substitution notifications, recently passing the NA Health and Welfare Committee's Legislative Subcommittee review, the Korean Medical Association readily voiced its opposition. This amendment primarily expands the scope of post-substitution notific
- Company
- New ADC drug introduced…expands treatment options
- by Son, Hyung Min Aug 28, 2025 06:09am
- New global drugs are awaiting entry into Korea’s antibody-drug conjugate (ADC) market one after another. Following Daiichi Sankyo Korea's application for domestic approval of the ADC anticancer drug ‘Datroway,’ AbbVie Korea is also proceeding with the approval process for its ovarian cancer-targeted ADC ‘Elahere.’ The industry anticipates t
- Policy
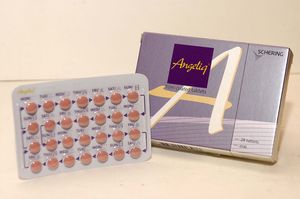
- Reimb listing approved for the first generic of 'Angeliq'
- by Lee, Tak-Sun Aug 27, 2025 06:07am
- The first generic of Angeliq Tab (drospirenone·estradiol, Bayer), a hormone-based medicine for use in postmenopausal women, will be included in the reimbursement listing. Analysis suggests that the commercialization of domestically produced generic is significant, considering that there had been supply issues related to Angeliq, which is
- Company
- Jaypirca’s reimbursement imminent in Korea
- by Eo, Yun-Ho Aug 27, 2025 06:07am
- The BTK inhibitor Jaypirca is likely to be listed for reimbursement soon. According to Dailypharm coverage, the National Health Insurance Service (NHIS) and Lilly Korea recently completed price negotiations for Jaypirca (pirtobrutinib), a treatment for relapsed or refractory mantle cell lymphoma (MCL). As a result, Jaypirca’s reimburs
- Company
- Targeted therapies and antibody drugs enter clinical trials
- by Son, Hyung Min Aug 27, 2025 06:07am
- Korea’s pharmaceutical and biotech industry is accelerating new drug development for pancreatic cancer, a cancer regarded as one of the most intractable cancers. Onconic Therapeutics, Prestige Biopharma, and Aptamer Sciences have each started clinical trials, making their bid into the field. The company plans to test the commercial viability of
- Company
- Co-promotion discussed for the diabetes drug Mounjaro
- by Kim, Jin-Gu Aug 27, 2025 06:07am
- The possibility of a co-promotion partnership between Eli Lilly and Korean pharmaceutical companies for 'Mounjaro,' the treatment of diabetes and obesity, continues to be discussed. While Lilly Korea officially maintains a 'direct sales' policy, it is reported that they are also conducting undiscloed discussions for potential partnerships.
- Policy
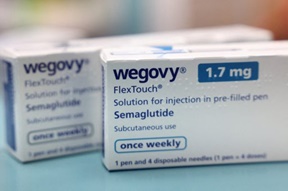
- ‘80,000 Wegovy prescriptions issued per month’
- by Lee, Jeong-Hwan Aug 27, 2025 06:06am
- The popular obesity drug Wegovy, which was launched in Korea in October last year, has already been prescribed about 400,000 times within eight months, raising concerns over potential misuse and abuse. On the 25th, Rep. Kim Sun-min of the Rebuilding Korea Party disclosed the ‘Annual and Monthly Status of Wegovy Prescriptions based on DUR