- Company
- Ferring Korea appoints Min-Jung Kim as new GM
- by Son, Hyung-Min May 17, 2024 05:47am
- On May 16, Ferring Pharmaceuticals Korea announced that the company appointed Min-Jung Kim as its new general manager, effective as of May 1. The new GM joined the company in 2021 as Chief Financial Officer (CFO). Kim has over 20 years of experience in finance, business development, SCM, IT, and human resources at global pharmaceutical an
- Policy
- Daewoong develops 'Olumiant' generic…starts clinical trials
- by Lee, Hye-Kyung May 17, 2024 05:47am
- Daewoong Pharmaceutical has started developing product to compete against Lily Korea’s JAK inhibitor 'Olumiant (baricitinib).' Olumiant was approved by the Ministry of Food and Drug Safety (MFDS) in December 2017. It is used for treating adult patients with moderate-to-severe rheumatoid arthritis who do not respond well to disease-modi
- Company
- Repotrectinib receives orphan drug designation in Korea
- by Eo, Yun-Ho May 17, 2024 05:47am
- , The &160;ROS1-targeted lung cancer drug repotrectinib received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) announced the designation in a recent announcement. Specifically, the indications repotrectinib received orphan drug designation for are as follows: ▲ treatment for patients with ROS1-posit
- Company
- Chinese Pharmas show presence at BIO Korea 2024
- by Moon, sung-ho May 16, 2024 05:48am
- Chinese pharmaceutical companies are starting to enter the domestic pharmaceutical and biotechnology market in earnest. This was the main observation made by the Korean industry at ‘BIO Korea 2024,’ cohosted by the Ministry of Health and Welfare and the Korea Health Industry Development Institute. The activities of major Chinese phar
- Company
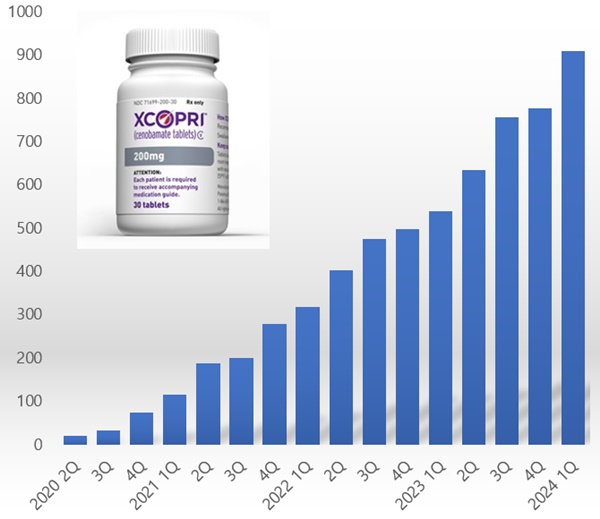
- Xcopri’s cumulative revenue surpasses KRW 1 trillion
- by Chon, Seung-Hyun May 16, 2024 05:48am
- Xcopri, SK Biopharmaceutical's new epilepsy drug, is continuing to show strong growth in the US market. Since its launch, sales have continued to rise every quarter, with cumulative sales exceeding KRW 600 billion. When including the revenue it had collected by licensing-out Xcopri’s technology, the company has secured more than KRW 1 trillion
- Opinion
- [Reporter’s View] Drug Review Coordination Council
- by Kim, Jin-Gu May 16, 2024 05:48am
- The Ministry of Food and Drug Safety (MFDS) has launched the Drug Approval and Review Coordination Council. The council will directly receive coordination requests from complainants when matters for supplemental measures arise during the drug approval and review process. With the Director-General of the Drug Safety Division heading the co
- Company
- "Camzyos, reimbursement would benefit gov and patients"
- by Eo, Yun-Ho May 16, 2024 05:48am
- The reason for delayed drug development in a particular disease typically falls into one of two. It’s either low awareness of the disease or challenges to drug development. Despite falling into these two categories, 'Camzyos (mavacamten)' was developed. It is a first-in-class targeted treatment option for obstructive hypertrophic cardi
- Policy
- Multiple sclerosis drug Ocrevusis approved in Korea
- by Lee, Hye-Kyung May 14, 2024 05:48am
- The Ministry of Food and Drug Safety (Minister: Yu-Kyung Oh) announced on the 13th that it has approved Roche Korea’s orphan drug Ocrevus (ocrelizumab) for multiple sclerosis (MS) in Korea. Multiple sclerosis is a chronic condition that develops in the central nervous system, which consists of the brain, spinal cord, and optic nerves and
- Policy
- BIO KOREA 2024 concludes a success
- by Lee, Hye-Kyung May 14, 2024 05:48am
- BIO KOREA 2024, cohosted by the Korea Health Industry Development Institute (President: Soon-do Cha) and the Provincial Administration of Chungcheongbuk-do (Governer: Young-hwan Kim), concluded successfully on the 10th. BIO KOREA 2024, which celebrates its 19th anniversary this year, was held for 3 days at COEX in Seoul under the theme of 'T
- Opinion
- [Reporter’s View] MFDS’ Regulatory Innovation 3.0
- by Lee, Hye-Kyung May 14, 2024 05:48am
- The Ministry of Food and Drug Safety (MFDS)’s announcement of the regulatory innovation tasks is now an annual event. The Regulatory Innovation 1.0, announced just two months after Oh Yu-kyoung’s appointment as the minister, focused on regulations that need system improvements. Since 1.0 was criticized for not considering citizens’ opini