- Opinion
- [Reporter’s View] Delays in reimbursement listing
- by Lee, Tak-Sun Apr 9, 2024 05:50am
- Last January, the Health Insurance Review and Assessment Service (HIRA) posted a document explaining the delay in listing and related press reports. The message was that the HIRA strives to implement faster listing, but pharmaceutical companies must cooperate. “Pharmaceutical companies must submit relevant documents for drugs subject
- Company
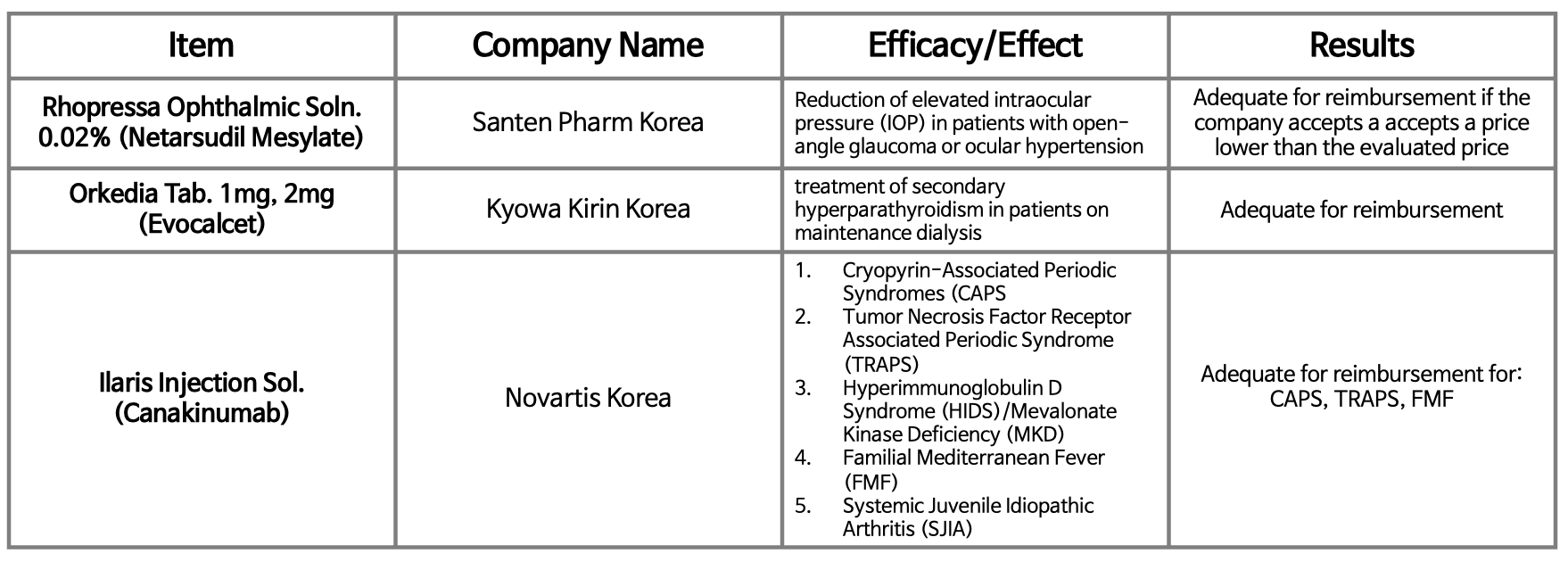
- Ilaris reimb passes DREC review, but again with a condition
- by Eo, Yun-Ho Apr 9, 2024 05:50am
- The orphan drug 'Ilaris' has again received a conditional reimbursement decision in Korea. Ilaris (canakinumab), Novartis Korea’s treatment for hereditary periodic fever syndrome, was quickly resubmitted to the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee on the 4th after receiving a conditiona
- Company
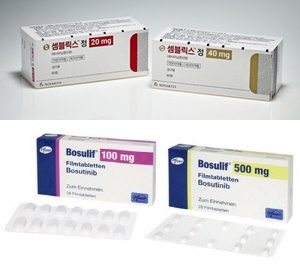
- Two new CML drugs were granted reimbursement in one year
- by Eo, Yun-Ho Apr 8, 2024 05:46am
- The chronic myelogenous leukemia (CML) treatment market is starting to show activity again. According to industry sources, treatment options for CML have been expanded with the reimbursement of Novartis Korea's 4th generation CML treatment ‘Scemblix (asciminib) in July last year, and Pfizer Korea’s 2nd generation drug 'Bosulif (bosutini
- Company
- Early breast cancer treatments fail to get reimb in KOR
- by Eo, Yun-Ho Apr 8, 2024 05:46am
- It seems unlikely that a new reimbursed option will be introduced to the early breast cancer environment anytime soon. First, Lilly Korea again failed to overcome the barrier of the Health Insurance Review and Assessment Service's Cancer Disease Review Committee for its CDK4/6 inhibitor Verzenio (abemaciclib). This was the company’s seco
- Company
- Sanofi’s Dupixent sales skyrocket after expanded indication
- by Nho, Byung Chul Apr 8, 2024 05:46am
- Sanofi-aventis Korea’s Dupixent (dupilumab) has accomplished outstanding external growth in the market for asthma biologic treatments. Based on pharmaceutical drug sales performance, Dupixent generated sales of KRW 143.1 billion last year. It has been ranked as the top-selling drug for the past five years. Dupixent sales were aroun
- Policy
- 'Need patient engagement for reimb of high-priced drugs'
- by Lee, Tak-Sun Apr 8, 2024 05:46am
- A study has shown that there is a need for a formal process for patient organizations and patients to participate in discussions for the reimbursement of high-priced drugs in Korea. With the reimbursement of high-priced drugs rising as a social issue and patient organizations and others raising concerns, the opinion has risen on the need for
- Policy
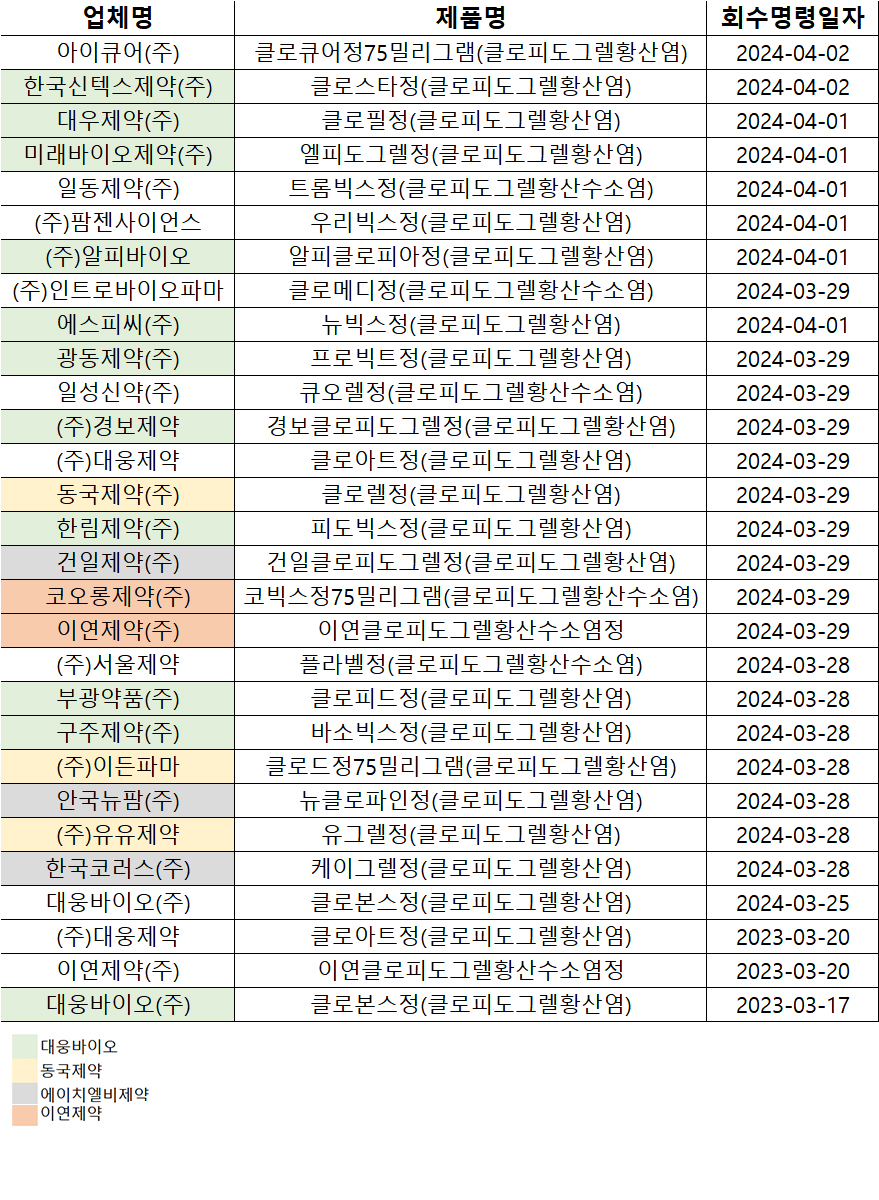
- Recalls of antiplatelet drugs containing 'clopidogrel'…
- by Lee, Hye-Kyung Apr 5, 2024 05:44am
- The recall of the products due to exceeding safety standards for miscellaneous impurities in safety tests for the antiplatelet drugs containing the ingredient 'clopidogrel' is expanding. The Ministry of Food and Drug Safety (MFDS) reported that a total of 29 items have been recalled until April 2, starting with Daewoong Bio’s 'Clovons Tab'
- Company
- High dose Eylea approved in KOR… extends dosing interval
- by Son, Hyung-Min Apr 5, 2024 05:43am
- Bayer Korea announced that its Eylea 8mg, a treatment for macular degeneration, was approved in Korea on the 3rd. Eylea 8mg was developed to maintain the effective drug concentration in the eye longer than the already approved Eylea 2mg product, allowing for longer dosing intervals and fewer injections. Eylea is an intravitreal inject
- Company
- AbbVie Korea, 52% increase in sales last year…
- by Kim, Jin-Gu Apr 5, 2024 05:43am
- AbbVie Korea’s major performances, including sales and operating profit, have increased by about 50% in a year. The analysis attributes this to the integration with Korea’s Allergan. AbbVie headquarters initiated the integration process between both companies in June 2019. The analysis suggests that the company’s total assets have ex
- Policy
- Orphan drug Ilaris receives conditional pass for reimb again
- by Lee, Hye-Kyung Apr 5, 2024 05:43am
- Although the government restarted reimbursement discussions for Novartis Korea's orphan drug Ilaris Inj (canakinumab) after 2 months, the results were the same. According to the "Results of the 4th 2024 Drug Reimbursement Review Committee Deliberations," which was released on the 4th by the Health Insurance Review and Assessment Service, Ilar