- Company
- Luxturna prescriptions available for rare retinal disease
- by Eo, Yun-Ho Mar 22, 2024 06:06am
- Prescriptions for the gene therapy ‘Luxturna,’ which targets only a few patients, are becoming available in South Korea. According to industry sources, Novartis Korea’s Luxturna (voretigene neparvovec), a therapy used to treat inherited retinal dystrophy (IRD), has cleared the Drug Committee (DC) of the tertiary general hospitals, in
- Policy
- Enhertu’s price falls to KRW 70 mil range with reimb
- by Lee, Tak-Sun Mar 22, 2024 06:06am
- The list price of the petitioned anti-cancer drug Enhertu was reportedly set at KRW 1.4 million per vial. This is significantly cheaper than the non-reimbursed price of KRW 2.3 million. In addition, the National Health Insurance Service (NHIS) will apply a risk-sharing agreement (RSA) system for the reimbursement of the drug, so the actua
- Policy
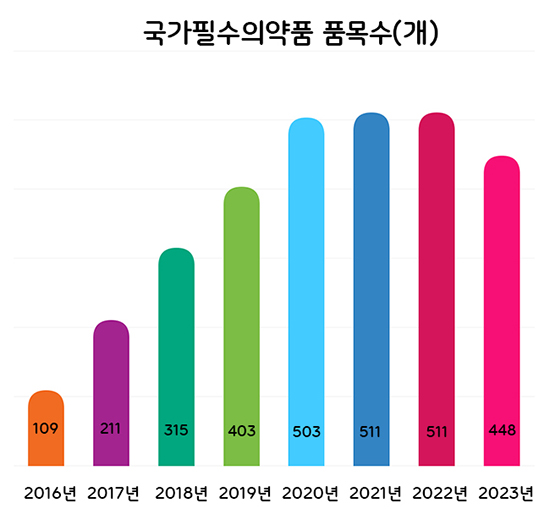
- National Essential Medicines to be categorized into 4 groups
- by Lee, Hye-Kyung Mar 21, 2024 05:53am
- The Ministry of Food and Drug Safety (MFDS) plans to categorize national essential drugs into 4 groups and conduct regular level assessments to better manage the drugs. According to the 'Research Service to Prepare a Measure to Classify and Manage the National Essential Medicine ‘ that was recently announced by MFDS, the authorities will dis
- Company
- "Envlo shows superior effects in diabetic kidney disease"
- by Son, Hyung-Min Mar 21, 2024 05:52am
- Daewoong Pharmaceutical announced on the 19th that Envlo (ingredient: enavogliflozin), an SGLT-2 class diabetes treatment from the company’s drug discovery, demonstrated superior effects in patients with diabetes compared to dapagliflozin. Based on the clinical results, Envlo has shown superior effects in all four indexes, including ▲
- Company
- Haleon Korea declares full independence from GSK
- by Eo, Yun-Ho Mar 21, 2024 05:52am
- The consumer healthcare company Haleon has declared its full independence from GSK. Haleon Korea, the Korean subsidiary of Haleon, announced on the 20th that it has completed the official registration of its business and is embarking on a new journey. The company had been completely spun off from GSK in July 2022 to become a 100% consu
- Company
- Fintepla gets orphan drug designation for Dravet syndrome
- by Eo, Yun-Ho Mar 21, 2024 05:52am
- 'Fintepla' has been designated as an orphan drug in South Korea for its indication for Dravet syndrome. The Ministry of Food and Drug Safety (MFDS) has announced this in the posting of the Orphan Drug Designation. UCB’s Fintepla (fenfluramine) initially acquired FDA approval for the treatment of rare epilepsy of infancy in 2020. Addit
- Policy
- Gov’t raises price of Harmonilan due to unstable supply
- by Lee, Tak-Sun Mar 21, 2024 05:52am
- The insurance ceiling price of the enteral nutrition formula Harmonilan (B Braun Korea), which the company has been experiencing difficulties in ensuring supply and demand recently, is expected to rise from April. The company had continuously been applying to register the drug as a ‘drug shortage prevention drug' and preserve productio
- Policy
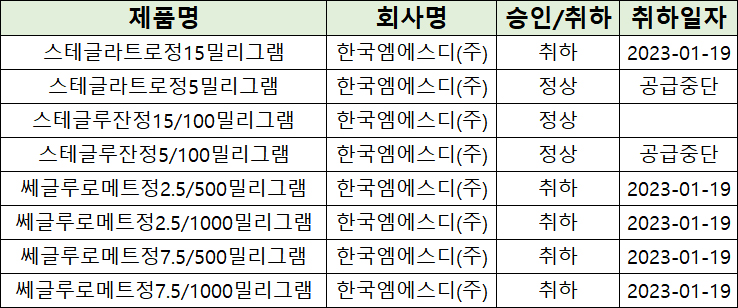
- MSD withdraws SGLT2 inhibitors in a row
- by Lee, Hye-Kyung Mar 20, 2024 05:44am
- MSD Korea is facing tough competition in the market for SGLT-2 inhibitors in South Korea. MSD decided that they will discontinue the supply of Steglatro Tab 5 mg and 'Stegluzan Tab 5/100 mg (ertugliflozin plus sitagliptin)' after voluntarily withdrawing 'Steglatro Tab 15 mg (ertugliflozin)' and metformin combination therapy, 'Segluromet,' la
- Policy
- President Yoon stands on expanding quota to 2,000
- by Lee, Jeong-Hwan Mar 20, 2024 05:44am
- President Yoon Suk Yeol reaffirmed the government’s plan to increase the medical school enrollment quota to 2,000 and stated, “Korea’s policy related to the number of doctors is not meeting the standards of the current era and actual needs, repeating the history of failure. Doctors’ licenses should not be used as a tool to intimidate K
- Policy
- Essential medical devices will be designated by next year
- by Lee, Hye-Kyung Mar 20, 2024 05:44am
- The Ministry of Food and Drug Safety is considering establishing a system to designate national essential medical devices. More specifically, the government plans to set a specific scope of medical devices that are essential for healthcare, such as rapid antigen test kits and life support devices that were necessary during COVID-19 but were diff