- Company
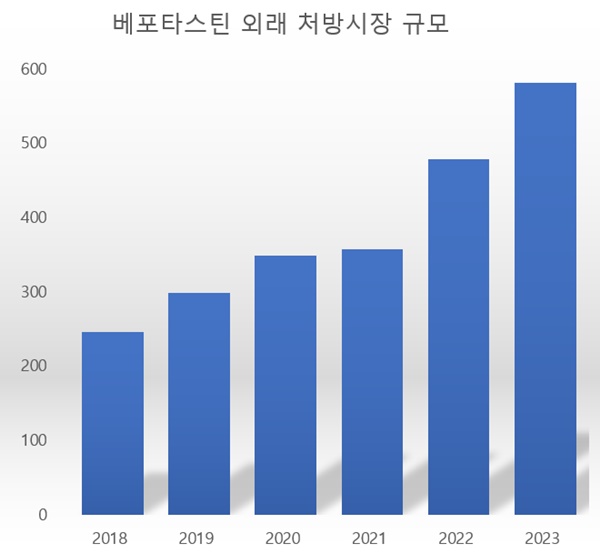
- Cash cow ‘bepotastine’ to face reimb re-evaluation
- by Chon, Seung-Hyun Mar 6, 2024 06:02am
- In facing re-evaluation of ‘bepotastine’ for reimbursement in the upcoming year, pharmaceutical companies fear its impact. The market size for bepotastine has increased by more than 50% in just two years during the pandemic and endemic, labeling bepotastine as a new cash cow. However, there are concerns that the companies may face financial lo
- Company
- Dupixent, 1st biologic approved for pruritic rash indication
- by Son, Hyung-Min Mar 5, 2024 05:49am
- As Dupixent is approved to treat prurigo nodularis (nodular itchy rash), the drug emerged as the only available treatment option among biologic agents. Previously, there were limited treatment options for treating prurigo nodularis, a condition that causes extreme itchiness. Sanofi hosted a press conference at Novotel Ambassador Seoul
- Product
- KPA, “to discuss ingredient prescribing in upcoming FAPA"
- by Kim JiEun Mar 5, 2024 05:49am
- Kwang-Hoon Choi, President of the Korean Pharmaceutical Association (KPA), has announced that he would assign the proposal for active ingredient prescribing as the priority agenda for the Federation of Asian Pharmaceutical Associations (FAPA) Congress, which is scheduled to be held in October this year. Choi aims to convince the Korean citi
- Company
- Kolon Life Science appeals to the Supreme Court for Invossa
- by Nho, Byung Chul Mar 5, 2024 05:49am
- On the 28th, Kolon Life Sciences decided to appeal to the Supreme Court against the manufacturing and sales license revocation ruling that had been made for its knee osteoarthritis cell gene therapy Invossa-K Inj (“Invossa”). In its appeal to the court, Kolon Life Sciences explained, "While we respect the court's decision, we will str
- Company
- Hugel receives FDA approval for its botulinum toxin Letybo
- by Nho, Byung Chul Mar 5, 2024 05:48am
- On the 4th, Hugel, a global total medical aesthetics company, announced that the company has received marketing approval from the U.S. Food and Drug Administration (FDA) on February 29th for 50 units and 100 units of its botulinum toxin Letybo (Korean brand name: Botulax). The FDA approval of Letybo represents a strong affirmation of Hu
- Company
- New multiple myeloma Ab ‘Elrexfio’ expects to enter KOR
- by Eo, Yun-Ho Mar 5, 2024 05:48am
- ‘Elrexfio,’ a new bispecific antibody to treat multiple myeloma, is expected to become commercially available soon. Pfizer Korea has applied for approval of Elrexfio (elranatamab) last year, and it is currently under review by the Ministry of Food and Drug Safety (MFDS), according to industry sources. Elrexfio is expected to be comme
- Policy
- Will Ferinject be reimbursed this time?
- by Lee, Tak-Sun Mar 5, 2024 05:48am
- Reimbursement for JW Pharmaceutical’s high-dose iron injection ‘Ferinject Inj’ is imminent in Korea. Although the company failed to pass the pricing negotiation stage once in 2020, analysts are seeing a true possibility for its reimbursement this time, as the company's willingness for its reimbursement is stronger than ever.
- Company
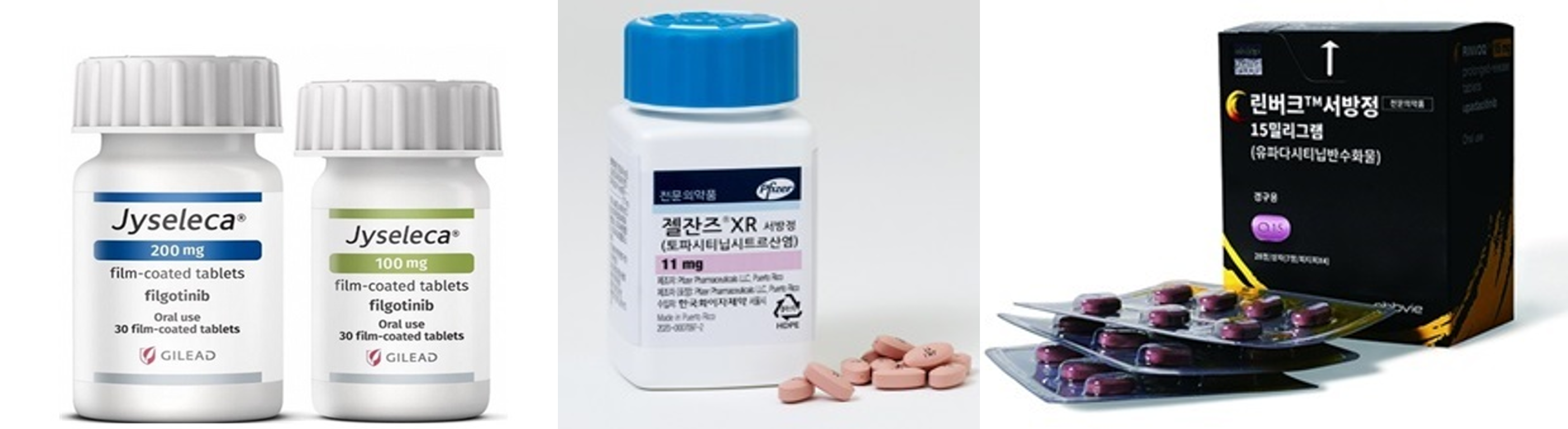
- Intensifying competition in ulcerative colitis drug market
- by Son, Hyung-Min Mar 4, 2024 05:53am
- New drug development for conquering ulcerative colitis drives competition among global pharmaceutical companies. Competition in the market will likely intensify due to the efficacy shown by new drugs, including JAK inhibitors Rinvoq and Xeljanz, anti-integrin drugs, and S1P receptor modulators, in clinical trials, in addition to biological medic
- Policy
- Guideline to be revised to prevent unintentional impurities
- by Lee, Hye-Kyung Mar 4, 2024 05:52am
- Amid the recent recall of sitagliptin combination products due to excess detection of impurities, the Ministry of Food and Drug Safety appears to be busy preparing a system to manage unintentional impurities at all times. The MFDS aims to revise the 'Guideline for Safety Management of Impurities in Drug Products’ within June to establis
- Company
- Generics of ‘Opsumit’ face tough competition in KOR
- by Kim, Jin-Gu Mar 4, 2024 05:52am
- In the market for the treatment of pulmonary arterial hypertension (PAH) with the active ingredient macitentan, the period of priority of sale given to the first generic drugs is set to expire on the first of next month. While Janssen’s ‘Opsumit’ competes with Samjin Pharm’s ‘Masiten’ in the market, Daewoong Pharmaceutical’s newly l