- Company
- Sobi, Handok’s partner has built competitive pipelines
- by Son, Hyung-Min Feb 16, 2024 06:03am
- Sobi, a biopharmaceutical company headquartered in Sweden, announced that it will launch its new drugs for treating rare diseases, including primary hemophagocytic lymphohistiocytosis (HLH), immune thrombocytopenia (ITP), and alkaptonuria (AKU), in Korea. Sobi and Handok have entered into a business agreement to launch in Korea. According to
- Policy
- Hanmi launches Zytiga generic combo after first generic
- by Lee, Tak-Sun Feb 16, 2024 06:00am
- Hanmi Pharmaceutical, which attracted wide attention by launching the first generic version of the prostate cancer treatment Zytiga last year, set out to target the market again by quickly developing a combination drug. On the 14th, the Ministry of Food and Drug Safety approved Hanmi Pharmaceutical's Abiteron Duo Tab 500/2.5mg. The
- Company
- GI makes bid with its new anticancer drug as combo therapy
- by Son, Hyung-Min Feb 16, 2024 06:00am
- Korean pharmaceutical bio venture company GI Innovation is working to identify the commercialization potential of its immuno-oncology drug in combination with an NK cell therapy. GI Innovation, which succeeded in licensing out its allergy drug candidate last year, plans to show results in the field of anticancer drugs this year. According
- Policy
- MFDS will inspect growth hormones and cold chains this year
- by Lee, Hye-Kyung Feb 16, 2024 05:59am
- The Ministry of Food and Drug Safety has included 'medical institutions and pharmacies handling growth hormone drugs' and 'cold chain compliance of high-risk items such as vaccines' in this year's planned inspections. The ' Biopharmaceuticals and Herbal Medicine Bureau’s Basic Plan for Manufacturing and Distribution Management in 2024,' whic
- Company
- Booming atopic dermatitis market, Adtralza nears reimb
- by Eo, Yun-Ho Feb 16, 2024 05:59am
- Adtralza, a treatment for atopic dermatitis, is expected to be the listed for insurance reimbursement. According to the industry, LEO Pharma has completed the price negotiations with the National Health Insurance Service (NHIS) for Adtralza (tralokinumab), a treatment for atopic dermatitis with an underlying mechanism of neutralizing in
- Policy
- ‘Trelegy 200 Ellipta’ concludes negotiations with the NHIS
- by Lee, Tak-Sun Feb 15, 2024 05:59am
- ‘Trelegy 200 Ellipta Inhaler,’ a triple combination therapy for COPD, has completed the negotiations with the National Health Insurance Service (NHIS). The drug is expected to be listed for health insurance reimbursement soon. According to industry experts on the 13th, ‘Trelegy 200 Ellipta Inhaler,’ a COPD triple combination therap
- Company
- ‘Concerned about Korea’s stroke treatment system’
- by Son, Hyung-Min Feb 15, 2024 05:58am
- The government announced the initiation of a pilot project to lay the foundation for a stroke care system, but its lack of content is being criticized by the academic society. Academics have suggested that securing human resources, establishing a compensation system, and revising the stroke disease group classification system should be made
- Company
- SK Chemical ‘will continue pharma business without selling'
- by Kim, Jin-Gu Feb 15, 2024 05:58am
- SK Chemicals made an official announcement on the 14th that it has decided not to pursue the sale of its pharmaceutical business. The company said, "Amid the various internal and external variables and a rapidly changing business environment, SK Chemicals has decided to maintain its current business portfolio and pursue stable operation
- Company
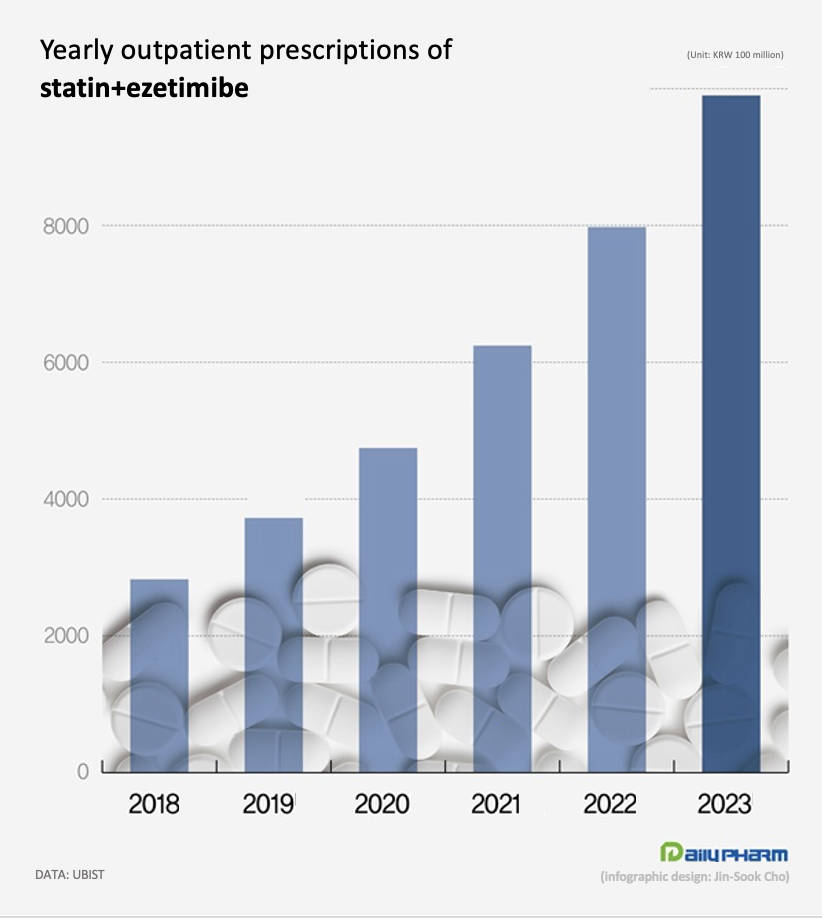
- Statin+ezetimibe combo mkt size nears ₩1 tril in KOR
- by Chon, Seung-Hyun Feb 15, 2024 05:58am
- The statin and ezetimibe combination continues to dominate the dyslipidemia treatment market. Prescriptions have more than tripled over the past 5 years, with the market size approaching nearly KRW 1 trillion. The rosuvastatin-ezetimibe combination drove market growth, posting more than KRW 600 billion in sales, while the atorvastatin-ezetimibe
- Policy
- The guidelines for PVA will be revised soon
- by Lee, Tak-Sun Feb 15, 2024 05:58am
- The National Health Insurance Service (NHIS) is likely to seek opinions from the pharmaceutical industry soon to revise the operational guidelines on the price-volume agreement (PVA) program. The revised guidelines draw attention as they include an agenda for easing on products that have reduced their prices more than three times in fiv