- Policy
- Price cut hold for Forxiga, Xigduo, Atmeg Combi are extended
- by Lee, Jeong-Hwan Feb 14, 2024 05:40am
- The administrative stay of execution of the price reduction disposition for AstraZeneca's diabetes drugs Forxiga (dapagliflozin), Xigduo (dapagliflozin+metformin), and Korea United Pharm’s Atmeg Combigel (atorvastatin 10 mg + omega 3) has been extended, and the insurance ceiling price for the drugs will remain unchanged until June 30.
- Policy
- Chinese MM drug Xpovio reattempts reimb listing in KOR
- by Lee, Tak-Sun Feb 14, 2024 05:40am
- A new drug for multiple myeloma that was developed by the Chinese pharmaceutical company Antengene is attempting reimbursement listing again in Korea. The drug’s name is Xpovio Tab 20mg (Selinexor). The drug received a non-reimbursement decision at the Drug Reimbursement Evaluation Committee meeting that was held in November last year.
- Company
- SK Chemical’s pharma biz recorded sales of 376.1 bil. won
- by Chon, Seung-Hyun Feb 14, 2024 05:40am
- SK Chemical's pharmaceutical business, which is to be sold, has reached its sales peak. The company's pharmaceuticals and new drugs under contract have demonstrated stable growth in the prescription market. On the 8th, SK Chemical reported that its pharmaceutical business generated sales of 376.1 won last year, representing an increase of 19.
- Company
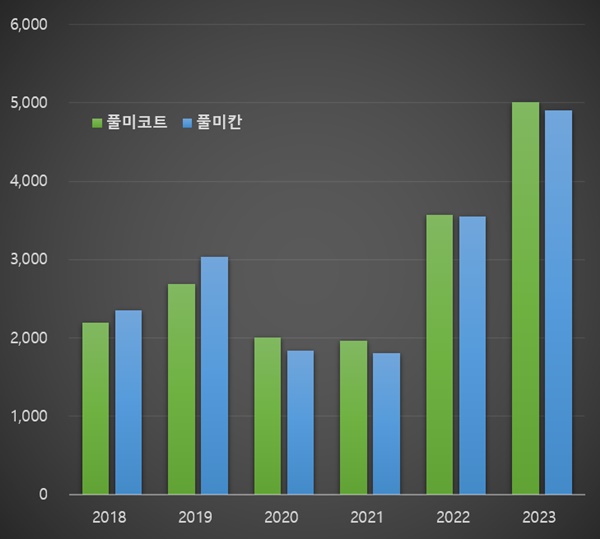
- Market for Pulmicort and Pulmican soars 2.6 times in 2 years
- by Chon, Seung-Hyun Feb 14, 2024 05:40am
- Last year, the prescription market for asthma medications containing the ‘budesonide’ ingredient expanded to its largest size ever. There have been significant demands for the treatment due to the circumstances surrounding the pandemic and endemic. At the end of last year, the drug pricing increase led to manufacturing and the prescription mar
- Policy
- Will Enhertu·Ilaris·morning sickness drugs be reimbursed
- by Lee, Tak-Sun Feb 14, 2024 05:40am
- Whether Enhertu Inj 100mg, Ilaris Injection Sol, and the morning sickness drugs that had passed the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee review on the 1st, will be listed for reimbursement in April is gaining attention. As all three drugs are highly sought-after by patients and the pub
- Company
- Unstoppable sales growth of Entresto
- by Kim, Jin-Gu Feb 13, 2024 06:18am
- Novartis Korea’s Entresto, a heart failure treatment, repeatedly shows significant growth. Entresto has consistently achieved over 30% growth in sales each year since its release in October 2017, having passed six years. Since its release, Entresto has undergone five price reductions to suppress the steep increase in prescription sales
- Policy
- HIRA to revise guidelines on new drugs utility assessment
- by Lee, Tak-Sun Feb 13, 2024 05:52am
- The Health Insurance Review and Assessment Service (HIRA) will revise the guidelines on indirect comparison for assessing the clinical utility of new drugs. For this purpose, HIRA is currently recruiting researchers for contract research work. On the 1st, the HIRA initiated public bidding for ‘Research services to revise guidelines on
- Policy
- Ulcerative colitis drug Omvoh is approved in Korea
- by Lee, Hye-Kyung Feb 13, 2024 05:51am
- The Ministry of Food and Drug Safety (MFDS, Minister: Yu-Kyoung Oh) announced that it has approved Lilly Korea's new drug Omvoh Inj (mirikizumab-mrkz, recombinant) for the treatment of ulcerative colitis on the 7th. Omvoh Inj. 20 mg/ml is a monoclonal antibody drug that binds to the p19 subunit of interleukin (IL)-23, providing a new th
- Policy
- Janssen’s Spravato required to submit domestic trial data
- by Lee, Hye-Kyung Feb 13, 2024 05:51am
- With Janssen’s novel drug for treatment-resistant depression (TRD), ‘Spravato Nasal Spray (esketamine hydrochloride)’ failed to demonstrate efficacy in Japanese and Chinese patients, the Korean authorities also held discussions on whether to maintain its marketing authorization status in Korea as well. The drug was approved in June 2020.
- Company
- ‘Biobetter Nexviazyme is a better treatment option'
- by Eo, Yun-Ho Feb 13, 2024 05:51am
- There are times when a single treatment has a significant impact on the overall management of the disease. This is especially true for rare and incurable diseases. In the field of metabolic diseases, a paradigm shift occurred when researchers found that certain rare patients lacked a single enzyme, which led to the development of therapie