- Company
- Vemlidy dominates stagnant 300 bil. won hepatitis B market
- by Son, Hyung-Min Jan 29, 2024 06:05am
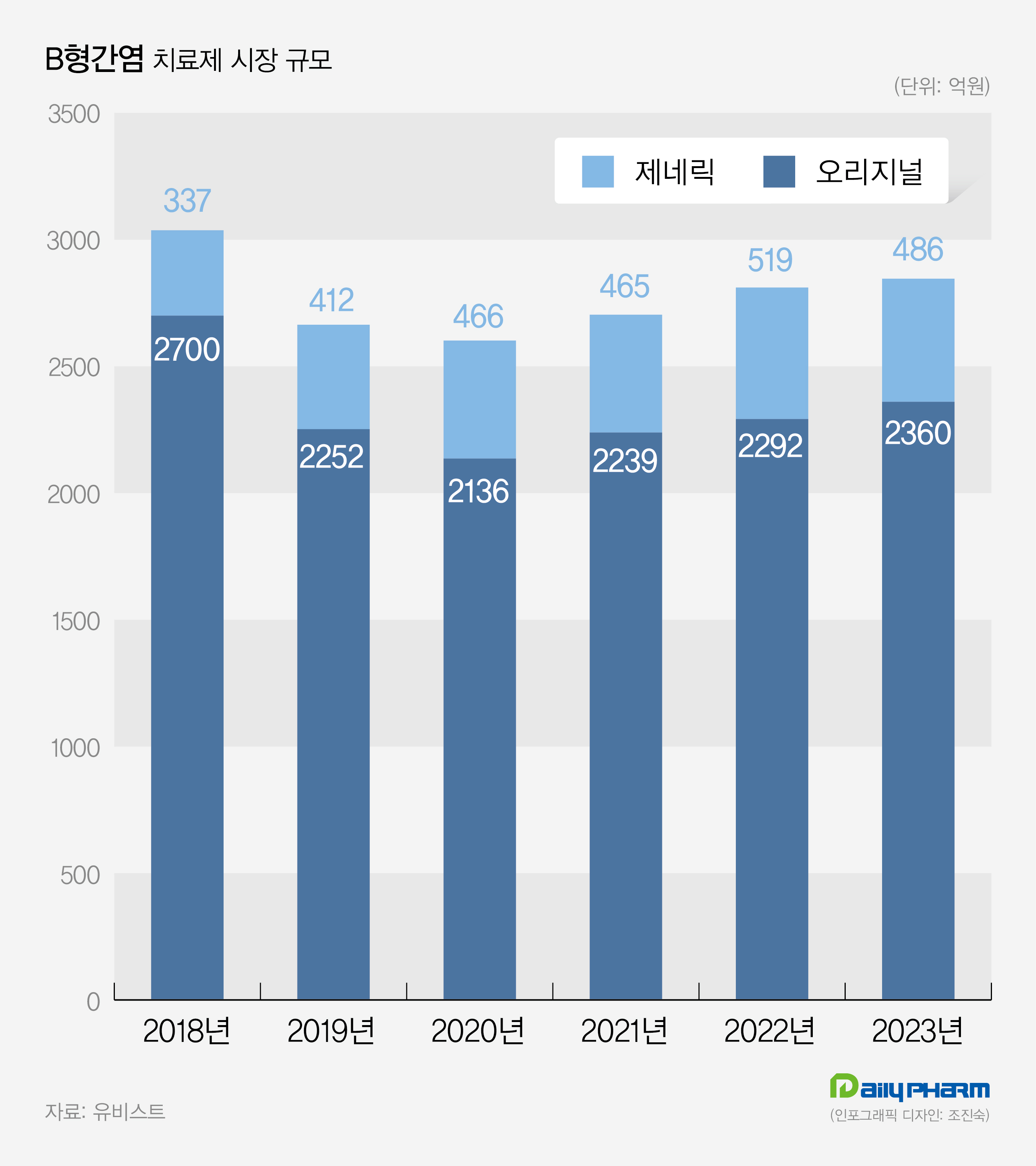
- The market for hepatitis B treatment, worth 300 billion won annually, is currently experiencing a slowdown. While Gilead Sciences Korea’s Vemlidy is expanding its market share, Viread and Baraclude are facing a decline. In the generics market, Dong-A ST is leading the market. Prescription sales for Vemlidy has increased by 26% compared t
- Policy
- Referencing lowest A8 price will result in a supply crisis
- by Nho, Byung Chul Jan 29, 2024 06:05am
- Health authorities and the pharma-bio industry are at an impasse over the implementation of 'A8 external reference pricing reassessments.’ Engaged in a tug-of-war, the two parties have difficulty finding common grounds. The Ministry of Health and Welfare, the Health Insurance Review and Assessment Service, the Korea Pharmaceutical and Bi
- Policy
- Remote GMP inspections not recognized from April
- by Lee, Hye-Kyung Jan 29, 2024 06:05am
- The GMP inspections, which were temporarily allowed non-face-to-face due to the difficulty of on-site evaluations during the COVID-19 outbreak, are gradually returning to ordinary procedures. After switching the pre-approval GMP item inspections back to full on-site inspections in December last year, the Ministry of Food and Drug Safety (
- Company
- Will Polivy secure reimbursement approval this year?
- by Eo, Yun-Ho Jan 29, 2024 06:04am
- The question of whether ‘Polivy,’ a B-cell lymphoma treatment, will be listed for insurance reimbursements this year is garnering significant attention. According to industry sources, Roche Korea’s Polivy (polatuzumab vedotin), a treatment for relapsed or refractory diffuse large B-cell lymphoma (DLBCL), is expected to be presented to
- Policy
- Lilly’s UC drug Omvoh is soon to be approved in KOR
- by Lee, Hye-Kyung Jan 26, 2024 05:51am
- Lilly's ulcerative colitis treatment Omvoh (mirikizumab-mrkz) is nearing approval in Korea. Omvoh received U.S. FDA approval in October last year and settled as the first and only interleukin-23p19 (IL-23p19) antagonist for the treatment of moderately to severely active ulcerative colitis (UC) in adults. According to industry sources,
- Policy
- P2T for LG Chem’s obesity drug LB54640 approved in Korea
- by Lee, Hye-Kyung Jan 26, 2024 05:51am
- LG Chem’s generic obesity treatment, 'LB54640,' has been approved for a Phase II clinical trial in Korea. On the 24th, the Ministry of Food and Drug Safety approved LG Chem’s application to initiate a randomized, placebo-controlled, double-blind Phase II study with an open extension period to evaluate the efficacy and safety of LB54640
- Company
- New CKD drug Kerendia lands in general hospitals in KOR
- by Eo, Yun-Ho Jan 26, 2024 05:51am
- The landing procedure for the new chronic kidney disease drug 'Kerendia' is in full swing in general hospitals in Korea ahead of its reimbursement listing. According to industry sources, Bayer Korea's Kerendia (finerenone) recently passed the drug committee (DC) review at Sinchon Severance Hospital. It is also undergoing a landing proces
- Opinion
- [Reporter’s View] Conglomerates expanding into the pharma
- by Kim, Jin-Gu Jan 26, 2024 05:51am
- M&A activities have made headlines in the pharmaceutical and biotechnology (pharma and biotech) industries in early 2024. Hanmi Pharmaceutical, a leader in the Korean pharmaceutical industry, has officially confirmed its merger with OCI group, a chemical company. Additionally, Orion has acquired LegoChem Biosciences, a globally recognized
- Policy
- MOHW “Drug pricing for listed drugs is set to be reduced"
- by Lee, Jeong-Hwan Jan 26, 2024 05:50am
- In February, the government will post the outcomes of notifications of the second-round review & assessment, analyzing the upper limit of standards and requirements associated with the drug pricing reduction of currently listed generics. The adjusted prices will take effect on March 1st. The drug pricing reduction related to market-bas
- Company
- Ildong Idience presents 1st interim results for Venadaparib
- by Kim, Jin-Gu Jan 25, 2024 05:50am
- On the 22nd, Idience, the new drug development subsidiary of Ildong Pharmaceutical, announced that they have presented the research findings related to 'Venadaparib' at the 2024 ASCO Gastrointestinal Cancers Symposium held from the 18th to the 20th. Venadaparib is a novel targeted anticancer candidate product with a mechanism focused on