- Policy
- Emerging candidate for Dir. of the Pharmaceutical Benefits
- by Lee, Jeong-Hwan Jan 19, 2024 05:45am
- Chang-Hyun Oh (55/College of Pharmacy, Chung-Ang University), the incumbent director of the Pharmaceutical Benefits at the Ministry of Health and Welfare (MOHW), is expected to be succeeded by Jung-min Yu (passed the 51st civil service exam/Ewha Womans University), who is currently serving as Secretary in the Office of the Senior Secretary
- Policy
- Celltrion rebrands and sells self-manufactured drugs
- by Lee, Hye-Kyung Jan 19, 2024 05:44am
- Celltrion Pharm will discontinue importing the original diabetes and hypertension drugs that it has successfully self-manufactured after acquiring all the rights, including sales rights and patents, from Takeda Pharmaceutical. According to the list of supply discontinuation drugs reported to the Ministry of Food and Drug Safety, the soon-
- Company
- Hemlibra reduces risk of exercise-associated bleeding
- by Lee, Seok-Jun Jan 19, 2024 05:44am
- The research findings, which include data on the physical activities of patients treated with JW Pharmaceutical’s hemophilia A treatment ‘Hemlibra (emicizumab)’ and their safety profile, have been published in the International Journal of Hematology (Int J Hematol). Hemlibra is an innovative new drug that mimics the function of blood
- Opinion
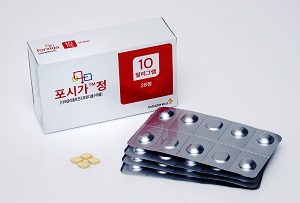
- [Reporter’s View] Forxiga withdrawal raises concern
- by Son, Hyung-Min Jan 19, 2024 05:44am
- AstraZeneca, the UK-based global pharmaceutical company, is withdrawing its diabetes treatment Forxiga (ingredient: dapagliflozin) from the Korean market, ten years since its approval in 2013 in Korea. Initially approved as SGLT-2 inhibitor-class diabetes treatment, Forxiga achieved success as its indications were expanded to include condi
- Company
- Keytruda's reimb for 6 indications will be reviewed
- by Eo, Yun-Ho Jan 19, 2024 05:44am
- The reimbursement challenge for Keytruda continues this year. Although no results have been made, the company seems to be on a steady course in applying for reimbursement. According to industry sources, 6 indications of MSD Korea’s PD-1 inhibitor immuno-oncology drug Keytruda (pembrolizumab) are expected to be submitted to the Health Ins
- Company
- AstraZeneca begins ERP and business unit restructuring
- by Son, Hyung-Min Jan 18, 2024 04:51pm
- AstraZeneca Korea has initiated an early retirement program following the withdrawal of the diabetes treatment Forxiga from the Korean market. The company has stated that it is in discussions with the government to minimize the impact of Forxiga’s withdrawal along with its business unit restructuring. According to the pharmaceutical industr
- Company
- Keytruda approved as 1st-line treatment for gastric cancer
- by Son, Hyung-Min Jan 18, 2024 04:46pm
- Keytruda, a cancer immunotherapy developed by MSD, has been approved as a first-line treatment for gastric cancer in Korea. Keytruda has emerged as a new first-line treatment option, thirteen years after Herceptin (ingredient: trastuzumab) received approval in 2010. On the 16th, MSD hosted a press conference at Lotte Hotel Seoul commenci
- Policy
- Moderna will only supply Spikevax Duo 2 in Korea
- by Lee, Hye-Kyung Jan 18, 2024 06:07am
- Moderna Korea has discontinued the supply of all other vaccines, leaving only its latest version, Spikevax Duo 2 (elasomeran, davesomeran) in circulation among the 5 COVID-19 vaccines it had received approval for in Korea. On the 17th, the Ministry of Food and Drug Safety (MFDS) received a report on Moderna’s supply discontinuation of ‘
- Company
- MA manager files complaint on NHIS official's abuse of power
- by Eo, Yun-Ho Jan 18, 2024 06:07am
- A controversy has arisen over the abuse of power made by an official from the National Health Insurance Service’s Department of Pharmaceutical Benefits. According to industry sources, B, a market access (MA) manager at pharmaceutical company A, filed a civil complaint with the Anti-Corruption & Civil Rights Commission’s e-People page, a
- Policy
- Drug price cuts will be applied in bulk next month
- by Lee, Tak-Sun Jan 18, 2024 06:07am
- A number of products are expected to receive price cuts next month as a result of the second round of reevaluations the government conducted on the insurance price ceiling of listed drugs. The drug price adjustments, which were initially set to be applied in January, were pushed back to February. Also, in consideration of the returns and