- Company
- K-pharma advances in new Alzheimer’s drug discovery
- by Jan 9, 2024 05:50am
- New drugs for treating Alzheimer’s disease developed by Korean pharmaceutical companies have entered the late phase of clinical trials, and industry watchers are closely monitoring their potential for commercialization. Aribio has recently submitted an Investigational New Drug (IND) application for its oral treatment candidate AR1001 for Alz
- Company
- GC Biopharma says its ‘Shingrix vaccine shows effect in P2T
- by Lee, Seok-Jun Jan 9, 2024 05:49am
- GC Biopharma (CEO Eun-cheol Heo) today announced positive Phase 2 results for its shingles vaccine, CRV-101 (ingredient name amezosvatein),’ which is being developed by its U.S. partner Curevo Vaccine. The data represent top-line results from a head-to-head comparison of GSK's market-leading shingles vaccine, Shingrix, and CRV-101, which
- Policy
- Data Exclusivity Bill for IMDs may soon be legislated
- by Lee, Jeong-Hwan Jan 9, 2024 05:49am
- A bill to amend the Pharmaceutica Affairs Act, which grants a 6-year data exclusivity for incrementally modified new drugs that have obtained domestic marketing authorization, was reviewed during the plenary session of the Legislation and Judiciary Committee that was held on the afternoon of the 8th. If approved by the committee, the b
- InterView
- ‘RCC treatment should reflect international guidelines’
- by Son, Hyung-Min Jan 9, 2024 05:49am
- The treatment paradigm for renal cell carcinoma has been changing with the emergence of immuno-oncology drugs, but the latest practice guidelines for the disease are not being reflected in practice in Korea. In-Keun Park, Professor of Oncology at Seoul Asan Medical Center stressed how treatment options for patients with recurrent renal ce
- Company
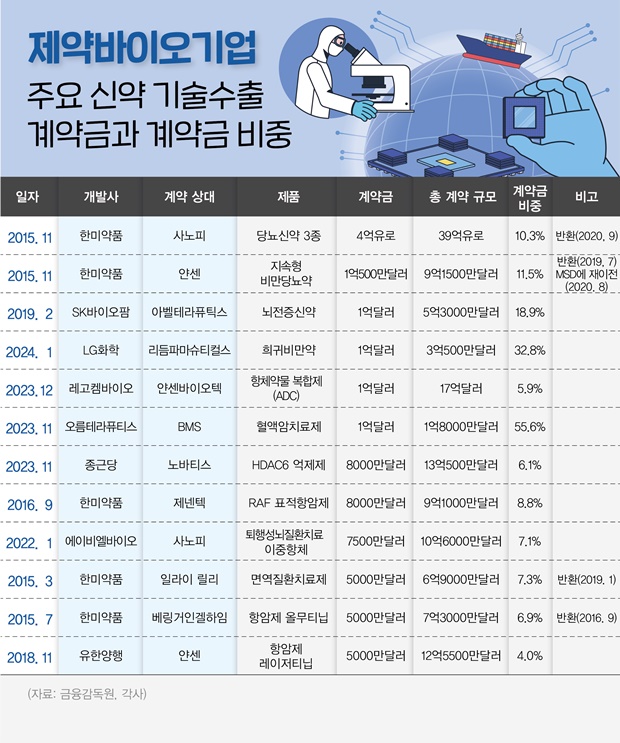
- Recent tech export deals boast record-high upfront payments
- by Chon, Seung-Hyun Jan 9, 2024 05:49am
- Since the end of last year, Orum Therapeutics, Chong Kun Dang Pharmaceutical (CKD Pharm), LegoChem Biosciences, and others have successfully secured large-scale technology transfer agreement with an upfront payment of 100 billion won. Out-licensing contracts with upfront payment scale over 10% of the total contract value have been on the rise du
- Company
- K-pharma to participate in J.P. Morgan Healthcare Conference
- by Jan 8, 2024 06:09am
- Korean pharmaceutical and biotech firms are fully prepared to attend the J.P. Morgan Healthcare Conference, the first international event of the year. The spotlight is on what accomplishments Korean pharmaceutical and biotech firms might achieve at this event, where they will join global companies to discuss large-scale technology transfe
- Company
- LG Chem transfers its rare obesity drug rights for KRW 130B
- by Kim, Jin-Gu Jan 8, 2024 06:09am
- LG Chem announced on the 5th that it had licensed out its new drug candidate that targets a rare genetic disease characterized by severe appetite control dysfunction to the U.S-based Rhythm Pharmaceuticals. The agreement, which amounts to USD 350 million (KRW 400 billion), includes an upfront payment of USD 100 million (KRW 130 billion
- Company
- Trodelvy can be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jan 8, 2024 06:09am
- Another new antibody-drug conjugate drug for breast cancer, ‘Trodelvy,’ can now be prescribed at general According to industry sources, Gilead Science Korea’s triple-negative breast cancer (TNBC) treatment ‘Trodelvy (sacituzumab govitecan-hziy)’ has recently passed the drug committee review of Seoul Asan Medical Center. In addit
- Opinion
- [Reporter's View] No regulatory innovation, no future
- by Son, Hyung-Min Jan 8, 2024 06:09am
- The importance of Decentralized Clinical Trials (DCT) through digital biomarkers in developing digital therapeutics (DTx) is gaining attention. Biomarkers are usually indicators used to detect changes within one’s body, using proteins, DNA, RNA, metabolites, etc. Digital biomarkers are an extension of this concept and refer to biomarkers
- Company
- GMP issues causing delays in new drug approval
- by Eo, Yun-Ho Jan 8, 2024 06:08am
- The GMP process, which inspects the manufacturing facilities for pharmaceutical products, has been identified as a contributing factor to the delay in the approval of pharmaceuticals. The Ministry of Food and Drug Safety (MFDS) has acknowledged the issue, but they have not yet addressed alternative solutions. Consequently, the bottleneck