- Company
- 'Tevimbra' applies for reimbursement for five indications
- by Eo, Yun-Ho Aug 13, 2025 06:07am
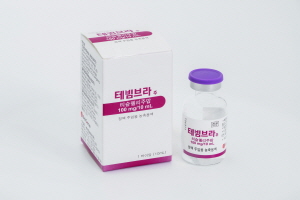
- 'Tevimbra' is quickly expanding following its successful inclusion in the insurance reimbursement for esophageal cancer. BeOneMedicines Korea has been confirmed to have applied for reimbursement on the day of approval for five new indications for its PD-1 inhibitor immunotherapy Tevimbra (tislelizumab), which received additional approval
- Policy
- New drug approval fees ₩410mil in KOR
- by Lee, Hye-Kyung Aug 13, 2025 06:07am
- Since the new drug approval fee was significantly increased to KRW 410 million starting this year, a total of 14 new products containing 10 ingredients have been submitted for approval. Although the specific product names cannot be disclosed, dedicated teams have been formed for 6 chemical drug substances and four biopharmaceutical substances, a
- Product
- Mounjaro set to hit shelves next week... pricing a dilemma
- by Jung, Heung-Jun Aug 13, 2025 06:07am
- With the imminent arrival of the diabetes and obesity treatment Mounjaro (tirzepatide) in Korea, pharmacies are struggling to set an appropriate price for the item. Due to the nature of high-priced non-reimbursable drugs, there are regional price differences, and price competition is expected in the future, so pharmacies are showing a
- Company
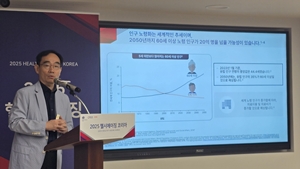
- Adult vaccination in a super-aged society highlighted
- by Whang, byung-woo Aug 13, 2025 06:07am
- South Korea has entered a super-aged society, with its population aged 65 and over exceeding 20%. The need for a response through adult vaccination is being emphasized. With the number of National Immunization Program (NIP) vaccines for adults limited to just influenza and pneumococcal vaccines, there is a growing opinion that the program nee
- Product
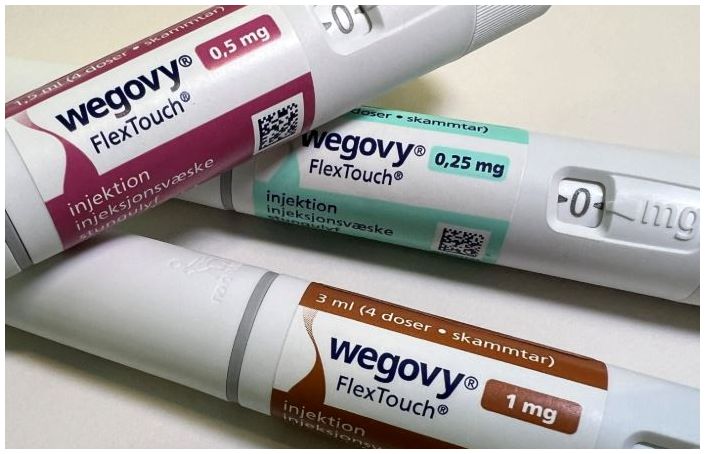
- Wegovy supply price cut by 40%... to counter Mounjaro
- by Kang, Hye-Kyung Aug 13, 2025 06:06am
- The supply price for Wegovy will be reduced by approximately 40% starting on the 14th. Refund measures are also expected to be implemented for pharmacies with inventory. Novo Nordisk notified pharmacies and other relevant parties of the changes through Julic and other channels. According to Dailypharm's coverage, the prices for four dosa
- Company
- Tariff impact inevitable for biodrugs’ export to the US
- by Cha, Jihyun Aug 12, 2025 06:16am
- Exports of domestically produced biopharmaceuticals to the US more than doubled last year compared to the previous year. If the Trump administration's tariffs on pharmaceuticals are implemented, the domestic biopharmaceutical export industry is expected to suffer significant damage. According to the “Key Data on the Domestic Biopharmaceutica
- Company
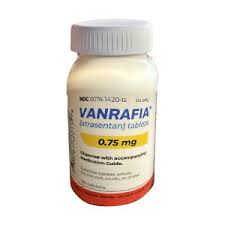
- 'Vanrafia' for rare kidney disease receives ODD in Korea
- by Eo, Yun-Ho Aug 12, 2025 06:16am
- A new drug for rare kidney disease, 'Vanrafia,' has been granted orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced this through notificiation board. The indication for the designation is to 'treat adult patients with primary immunoglobulin A nephropathy (IgAN) with a urine protein-to-creatin
- Opinion
- [Reporter's View] Sales dilemma of newly listed bioventures
- by Whang, byung-woo Aug 12, 2025 06:15am
- &160;“Off to a fair start” was the market’s assessment regarding the stock prices and performance of biotech companies that went public on the KOSDAQ this year. However, upon closer inspection, some point out that the strengthened scrutiny of financial figures following the Fadu incident (accounting and revenue recognition controvers
- Company
- US Biosecurity Act passing is ramping up
- by Kim, Jin-Gu Aug 12, 2025 06:14am
- The U.S. Congress is re-pursuing the Biosecurity Act, which failed to pass last year. The bill explains a comprehensive set of sanctions against companies designated as 'biotechnology companies of concern.' Analysis suggests that the bill's chances of passing have increased, as it addresses the procedural issues that were a stumbling bl
- Policy
- Smokers at 54.5 times higher risk of SCLC than non-smokers
- by Lee, Tak-Sun Aug 12, 2025 06:13am
- A study has found that 30-year smokers have a 54.5 times higher risk of developing small cell lung cancer than non-smokers. The National Health Insurance Service (NHIS) Health Insurance Policy Research Institute announced on the 11th the results of a comparative analysis of cancer incidence risk and attributable risk by cancer type among indi