- Reimb discussion on Braftovi and Luxturna continue next year
- by Eo, Yun-Ho Dec 18, 2023 05:31am
- Industry attention is focused on the fate of the two new drugs that were unable to pass the final stage to reimbursement in Korea. According to industry sources, Ono Pharma Korea’s colorectal cancer drug Braftovi (encorafenib) and Novartis' retinal disease drug Luxterna (voretigene neparvovec-rzyl) have entered into extended negotiations
- Company
- SK pneumococcal conjugate vaccine nears global entry
- by Chon, Seung-Hyun Dec 15, 2023 05:51am
- SK Bioscience is fast-tracking its global market strategy for its next-generation pneumococcal vaccine. Nine years following the start of their joint development with Sanofi, the company has now progressed to the last phase of clinical trials for commercialization. SK Bioscience has also been improving its vaccine production facilities to prepar
- Policy
- Global orphan drug Lamzede receives GIFT designation
- by Lee, Hye-Kyung Dec 15, 2023 05:51am
- 'Lamzede Inj (velmanase)’ received the Global Innovative Products on Fast Track (GIFT) designation and will receive an expedited review in Korea. Lamzede is an orphan drug for which Kwang Dong Pharmaceutical signed an exclusive sales and distribution agreement with Italy's Chiesi Farmaceutical in July this year. As the GIFT program see
- Policy
- HER2-positive metastatic breast cancer drug Tukysa approved
- by Lee, Hye-Kyung Dec 15, 2023 05:51am
- The Ministry of Food and Drug Safety (MInister: Yu-kyoung Oh) approved two dosage forms (50mg, 150mg) of MSD Korea’s new breast cancer drug Tukysa (tucatinib) on the 14th. The drug is used in combination with trastuzumab and capecitabine, for patients with locally advanced or metastatic HER2-positive breast cancer who have received two o
- Company
- Reimb for Cold Agglutinin Disease drug Enjaymo starts in KOR
- by Eo, Yun-Ho Dec 15, 2023 05:51am
- The cold agglutinin disease treatment ‘Enjaymo’ is seeking reimbursement listing in Korea. According to industry sources, Sanofi Korea has filed a reimbursement application for its Enjaymo (sutimlimab) as a treatment for hemolysis in adult patients with Cold Agglutinin Disease (CAD) Enjaymo’ is a first-in-class humanized monoclonal
- Company
- US-expanded Cresemba faces reimb challenges in KOR
- by Son, Hyung-Min Dec 15, 2023 05:51am
- The antibiotic Cresemba has received expanded indications in the US to include pediatric patients. However, in Korea, the reimbursement for Cresemba is being delayed because of challenges in economic evaluations, limiting patient access to this medication. According to the industry on the 15th, the U.S. Food and Drug Administration (FDA) appr
- Policy
- Non-inferior drugs likely to receive preferential pricing
- by Lee, Tak-Sun Dec 15, 2023 05:51am
- Non-inferior drugs from Korea innovative pharmaceutical companies will likely be eligible for preferential drug pricing starting next year. Previously, non-inferior drugs were priced below the weighted average of alternative drugs. However, through improvement measures, if the patent of an alternative drug is still active, non-inferior d
- Company
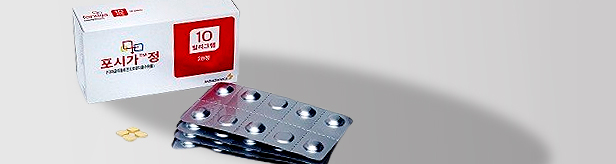
- Domestic market withdrawal of Forxiga remains a mystery
- by Kim, Jin-Gu Dec 14, 2023 05:47am
- AstraZeneca’s decision to withdraw its SGLT-2 inhibitor diabetes drug Forxiga (dapagliflozin) from the Korean market is being considered an unusual move by the industry. This is because the decision to pull out of the Korean market would essentially mean giving up KRW 50 billion in annual sales for AstraZeneca. While AstraZeneca's offi
- Company
- Rinvoq is granted reimb for ankylosing spondylitis
- by Son, Hyung-Min Dec 14, 2023 05:47am
- The use of JAK inhibitors, which had been used for rheumatoid arthritis and atopic dermatitis, has been expanded to ankylosing spondylitis. On the 13th, AbbVie Korea held a press conference at Andaz Seoul Gangnam in Gangnam-gu, Seoul to celebrate the reimbursement of its Janus kinase (JAK) inhibitor, Rinvoq (upadacitinib) for severe acti
- Company
- Reimb of latecomer leukemia drug Bosulif imminent
- by Eo, Yun-Ho Dec 14, 2023 05:47am
- Bosulif, a new leukemia treatment, is expected to receive reimbursement next year. According to the pharmaceutical industry, Pfizer Korea successfully completed drug pricing negotiations with the National Health Insurance Service for Bosulif (bosutinib), a Chronic Myelogenous Leukemia (CML) treatment. Bosulif will be presented to the