- Policy
- Will Enhertu be reimbursed using flexible ICER threshold?
- by Lee, Tak-Sun Dec 14, 2023 05:47am
- With the government reportedly planning to flexibly apply the Incremental Cost-Effective Ratio (ICER) threshold, whether the breast cancer treatment Enhertu (fam-trastuzumab deruxtecan, Daiichi Sankyo) will finally find its way to reimbursement is gaining attention in Korea. The drug’s reimbursement review is currently stuck in HIRA’s
- Company
- Daewoong attempts at first Lupron Depot generic in the US
- by Chon, Seung-Hyun Dec 13, 2023 05:38am
- Daewoong Pharmaceutical is seeking to enter the U.S. anticancer market with the global pharmaceutical company, Zydus Worldwide DMCC. Daewoong Pharmaceutical announced on the 11th that it had signed a licensing agreement with Zydus Worldwide DMCC. to co-develop and commercialize the anticancer drug DWJ108U Depot suspension injection. Th
- Policy
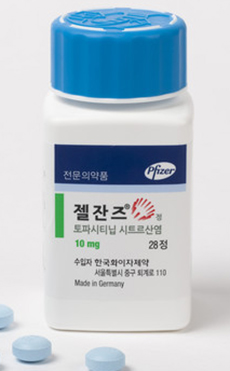
- Only CKD and Jeil have high-dose Xeljanz generics approved
- by Lee, Hye-Kyung Dec 13, 2023 05:38am
- Jeil Pharm received approval for its ‘Topazan Tab 10mg,’ a high-dose generic version of the rheumatoid arthritis treatment ‘Xeljanz (tofacitinib citrate).’ With the Ministry of Food and Drug Safety’s approval of Topazan Tab 10mg on November 11 in addition to the Topazan Tab 5mg that was approved on November 27, Jeil Pharm now owns b
- Opinion
- [Reporter’s View] Amid hopes for K-drugs, beware
- by Eo, Yun-Ho Dec 13, 2023 05:38am
- It is not only the patients that are now interested in the research and development of new drugs. The interest in high-value-added industry and aspirations for the development of the domestic industry has never been hotter. Industry development is good. New drugs? Even better. However, the rise of the stock market bubble that stirs inve
- Company
- Jeil will exclusively supply Norvatis’ 9 ophthalmic drugs
- by Nho, Byung Chul Dec 12, 2023 10:33pm
- Jeil is venturing into the field of ophthalmic diseases for the first time. Jeil Pharmaceutical (CEO Sung Suk-Je) announced on the 11th that it has signed an exclusive distribution agreement with Novartis Korea (CEO Yoo Byung-Jae) for the sale and supply of nine drugs in ophthalmic diseases including glaucoma and conjunctivitis. to targ
- Company
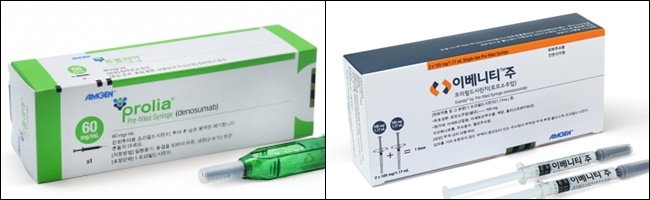
- Evenity and Prolia sales surge...combined sales rise 47%
- by Kim, Jin-Gu Dec 12, 2023 05:38am
- Amgen’s two osteoporosis treatments, Prolia (denosumab) and Evenity (romosozumab) are repeatedly posting high growth in sales. Together, these two treatments generated over 130 billion won in Q3 sales, dominating the osteoporosis treatment market in Korea. In the first-line, osteoporosis treatment is trending towards sequential Evenit
- Policy
- KIDS ‘Oral VEGFR-TKI use may increase risk of AAD’
- by Lee, Hye-Kyung Dec 12, 2023 05:38am
- The Korea Institute of Drug Safety & Risk Management (President: Jeong Wyan Oh, KIDS) announced that the use of Vascular Endothelial Growth Factor Receptor-Tyrosine Kinase Inhibitor (VEGFR-TKI) was associated with an increased risk of aneurysm and artery dissection occurrence. The findings were published in the internationally recognized
- Company
- AZ will withdraw its KRW 50 bil Forxiga from KOR mkt
- by Kim, Jin-Gu Dec 12, 2023 05:38am
- Forxiga (dapagliflozin), an SGLT-2 inhibitor class diabetes drug worth KRW 50 billion a year, will withdraw from the domestic market. According to industry sources on the 11th, AstraZeneca Korea recently made the decision to discontinue supply of Forxiga in Korea. However, the company will continue to supply its dapagliflozin and metfor
- Policy
- MOHW agrees to review current prior authorization system
- by Lee, Jeong-Hwan Dec 12, 2023 05:38am
- Medical professionals are raising concerns about significant disparities in approval rates of the 'rare diseases drug prior authorization system,' which differ by patients and by diseases, and the ambiguity of the criteria. The government appears to be failing to provide a direction for system improvement and address these concerns. Howev
- Company
- Reimb at a halt for one-shot IRD drug Luxturna
- by Eo, Yun-Ho Dec 12, 2023 05:38am
- Reimbursement discussions for the one-shot retinal disease treatment Luxturna is being delayed in Korea. According to Dailypharm coverage, Novartis Korea failed to reach an agreement with the National Health Insurance Service on the drug price of uxturna (voretigene neparvovec) for inherited retinal dystrophy (IRD) within the 60-day dea