- Company
- Gilead’s Livdelzi receives orphan drug designation in KOR
- by Eo, Yun-Ho Aug 11, 2025 06:05am
- The new primary biliary cholangitis drug ‘Livdelzi’ has received the orphan drug designation in Korea. The Ministry of Food and Drug Safety announced the news in a public notice on the 4th. Specifically, the designated indication is “the treatment of primary biliary cholangitis (PBC) in adults who have an inadequate response or intoler
- Company
- First TYK2i Sotyktu shows effect in Asian psoriasis patients
- by Son, Hyung Min Aug 11, 2025 06:04am
- The psoriatic arthritis treatment Sotyktu, which is jointly marketed in Korea by BMS Korea and Yuhan Corp, has established itself as a personalized oral treatment option for psoriatic arthritis patients in Korea, accumulating data from patients in East Asia. Sotyktu has been proven effective even in difficult-to-treat areas such as the scalp
- Company
- Plavix’s sales continue to grow 27 years into its release
- by Kim, Jin-Gu Aug 11, 2025 06:04am
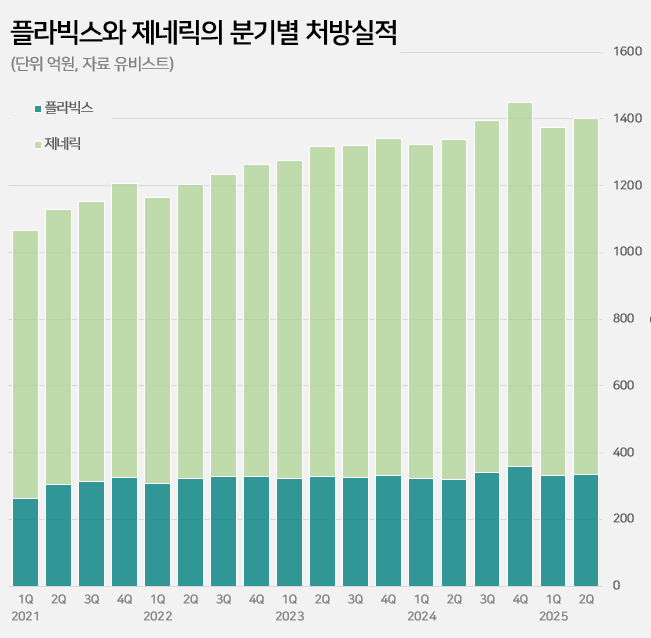
- Sales of the original drug ‘Plavix’ continue to rise in its 27th year of release in the clopidogrel-based antiplatelet agent market. It has maintained its lead with a 23% market share in the annual KRW 550 billion market. Meanwhile, major generic products are showing signs of slowing down. Samjin Pharmaceutical's ‘Platless’ saw a decr
- Company
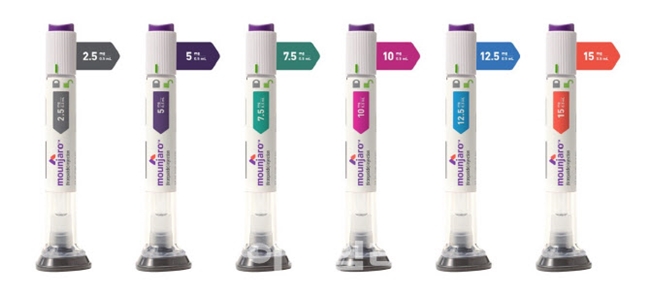
- The launch of Mounjaro is imminent
- by Moon, sung-ho Aug 11, 2025 06:03am
- The launch of Eli Lilly's 'Mounjaro,' which is called a 'miracle weight-loss drug' and garnered global attention alongside Wegovy, in Korea, is imminent. With the domestic launch of Mounjaro scheduled for mid-August, competition in the obesity treatment market, besides diabetes, is entering a new phase. There is significant interest in the
- Company
- New pulmonary hypertension drugs are competing for approval
- by Moon, sung-ho Aug 11, 2025 06:03am
- Global pharmaceutical companies are introducing new treatment options for pulmonary hypertension, utilizing novel mechanisms, which are being successively approved in the Korean market. These drugs are expected to change the clinical treatment paradigm. It's anticipated that they will establish a new market alongside the currently reimbursed
- Company
- Jaqbo’s substance patent extended to 2040
- by Kim, Jin-Gu Aug 8, 2025 06:03am
- Onconic Therapeutics announced on the 7th that the patent term for Jaqbo (zastaprazan), a new drug for gastroesophageal reflux disease in the P-CAB (potassium-competitive acid blocker class, has been extended by four years and two months. According to the company, the Korean Intellectual Property Office recently extended the patent term o
- Policy
- Will RSV vaccines be included in the NIP?
- by Whang, byung-woo Aug 8, 2025 06:03am
- With the RSV (respiratory syncytial virus) vaccine being released in Korea this year, interest in its inclusion in the National Immunization Program (NIP) has been gaining attention. According to the National Assembly's legislative information system, on the 6th, Rep. Yong-ki Jeon of the Democratic Party of Korea introduced a bill titled “Pa
- Company
- Rapid growth in RSV prevention market for infants
- by Whang, byung-woo Aug 8, 2025 06:02am
- After the COVID-19 pandemic, the importance of preventing infectious diseases has been re-emphasized. A new anti-RSV (Respiratory Syncytial Virus) antibody injection for infants, Beyfortus, is gaining attention as a potential turning point in the vaccine market Korea. Attention has been focused on whether a presidential pledge to support thi
- Company
- Immunosuppressant market rapidly expands in 3yrs in KOR
- by Kim, Jin-Gu Aug 8, 2025 06:02am
- The market for major immunosuppressants such as tacrolimus, cyclosporine, and mycophenolate has rapidly expanded over the past three years. The related market grew relatively slowly at less than 5% until 2022, but since 2023, it has expanded by more than 10% a year, showing a sharp growth trend. The tacrolimus and mycophenolate markets also s
- Company
- Mounjaro set for launch in Korea this month
- by Son, Hyung Min Aug 8, 2025 06:02am
- Eli Lilly Korea's diabetes and obesity treatment, Mounjaro, is set to launch in mid-August. Therefore, it will compete against Novo Nordisk's Wegovy. Lilly plans to handle Mounjaro's distribution directly, a strategy that differentiates it from Novo Nordisk, which is currently seeking co-promotion partners. According to the distribution indus