- Company
- Seo Jeong-jin, targets sales of 5 trillion won for new drugs
- by Kim, Jin-Gu Oct 26, 2023 06:06am
- Seo Jeong-jin, Chairman of Celltrion Group, presented a goal to increase global new drug sales to 5 trillion won by 2030, focusing on 'Zymfentra', which was recently approved as a new drug in the United States. Chairman Seo made this announcement at a press conference held at NH Investment & Securities in Seoul on the 25th. Previously,
- Policy
- Minister Cho will work to address shingles vaccine issue
- by Lee, Hye-Kyung Oct 26, 2023 06:06am
- The government responded that it would seek ways to ease the public burden in response to the request that shingles vaccines should be included in Korea’s National Immunization Program (NIP). Rep Young-Joo Kim of the Democratic Party of Korea pointed at the audit of the NA Health and Welfare Committee that was held on the 25th. She said,
- Policy
- Prostate cancer tx Xtandi·Zytiga, 5% pt copayment applied
- by Lee, Tak-Sun Oct 26, 2023 06:06am
- Mandatory coverage is applied to some treatments for prostate cancer treatments Xtandi soft capsule 40mg and Zytiga, which is expected to reduce the financial burden on patients. This is a measure of fairness as Erleada is subject to mandatory benefit as it was previously listed. The HIRA announced that it will revise the anticancer drug
- Policy
- Will restrictions be eased for vaccine clinical trials?
- by Lee, Jeong-Hwan Oct 26, 2023 06:06am
- Minister of Food and Drug Safety Yu-Kyung Oh promised to reduce or ease the mandatory participation rate of domestic clinical patients from the current 10% to 5% to strengthen sovereignty over domestically produced vaccines and increase self-sufficiency. The plan is to improve the current regulations by accepting the claims raised by dome
- Company
- Verzenio reattempts reimb for early breast cancer in KOR
- by Eo, Yun-Ho Oct 26, 2023 06:06am
- Verzenio, the first CDK4/6 inhibitor to attempt reimbursement listing for early breast cancer, has begun its second journey toward reimbursement in Korea. This attempt is being made 5 months after the first failure. The news of Lilly Korea's Verzenio (Abemaciclip) reapplication for reimbursement was made known through a written inquiry ma
- Company
- Janssen ‘will continue endeavors to the Korean society’
- by Son, Hyung-Min Oct 25, 2023 11:08am
- Janssen, which has succeeded in developing treatments in various areas including mental health, autoimmune diseases, and oncology over the past 40 years, emphasized that it will continue to contribute to Korean society by providing innovative medicines and fostering talent. On the 23rd, the company held a press conference celebrating its
- Company
- JW Pharma terminates contract after 5 yrs of atopy new drug
- by Chon, Seung-Hyun Oct 25, 2023 05:25am
- JW Pharma announced on the 20th that the technology transfer contract for 'JW1601', a treatment for atopic dermatitis, signed with Danish pharmaceutical company Leopharma, has been terminated. The company explained, “We have received a notice of termination of the contract from the Leopharma side and have agreed to return all rights.” JW1
- Company
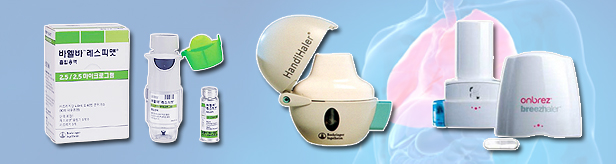
- Combo drugs strong in the COPD treatment market
- by Son, Hyung-Min Oct 25, 2023 05:24am
- The growth of the combination drugs was notable in the chronic obstructive pulmonary disease (COPD) treatment market in Q3 this year. With major COPD treatment guidelines recommending a long-acting muscarinic antagonist (LAMA) and long-acting beta2 agonist (LABA), the prescription trend appears to have shifted toward combination drugs. In partic
- Opinion
- [Reporter’s View] Asthma drugs reimb through diff tracks
- by Eo, Yun-Ho Oct 25, 2023 05:24am
- An unusual case has emerged where drugs for the same indication were listed through different tracks for reimbursement in Korea. The interleukin-5 antagonists for asthma, ‘Cinquair (reslizumab)’ and GSK Korea’s ‘Nucala (mepolizumab)’ have been simultaneously listed for reimbursement in Korea through different reimbursement tracks. Ci
- Company
- Hanmi’s P3T for ’Korean GLP-1 obesity drug’ is approved
- by Lee, Seok-Jun Oct 25, 2023 05:24am
- The development of an ‘GLP-1 obesity treatment optimized for Koreans' is gaining momentum. Hanmi Pharmaceutical announced on the 23rd that the Ministry of Food and Drug Safety approved the Phase III clinical trial for its ‘efpeglenatide,’ a glucagon-like peptide 1 (GLP-1) receptor agonist developed by the company. Epeglenatide is a