- Company
- LG Chem-BR Pharm signs MOU for HP Vitaran in CHN
- by Lee, Seok-Jun Oct 20, 2023 05:31am
- On the 19th, LG Chem announced it had signed a Memorandum of understanding (MOU) with BR Pharm, a regenerative medicine technology research and manufacturing company, for the development and approval of its PN (polynucleotide) skin booster 'HP Vitaran' in China. BR Pharm's 'Vitaran', which LG Chem introduced to the Korean market in Septe
- Company
- Gilead’s TNBC drug Trodelvy is released in KOR
- by Son, Hyung-Min Oct 20, 2023 05:31am
- Gilead Sciences Korea announced on the 18th that it has launched its metastatic triple-negative breast cancer drug ‘Trodelvy (sacituzumab govitecan)’ in Korea. Until now, Trodelvy has been supplied through the Korea Orphan & Essential Drug Center, but from the 18th, Gilead will supply it domestically, and patients will be able to use
- Opinion
- [Reporter's view]Skill is more important than trends
- by Oct 20, 2023 05:31am
- Recently, antibody-drug conjugates (ADCs) have emerged rapidly in the pharmaceutical industry. ADC is a biopharmaceutical that combines an antibody that binds to a specific target antigen on the surface of cancer cells and a drug (payload) with a powerful cell-killing function. Unlike Roche Kadcyla, which is classified as a first-generati
- Policy
- Will a new Alzheimer’s drug be introduced in 20 yrs in KOR?
- by Lee, Tak-Sun Oct 20, 2023 05:31am
- New Alzheimer's disease treatments are accelerating their introduction into the country. Following Eisai's application for the approval of its ‘lecanemab’ in June, Lilly also received approval from the Ministry of Food and Drug Safety to initiate a multinational Phase 3 clinical trial for ‘donanemab’ in Korea. As Biogen applied to
- Opinion
- [Reporter's view]Promotion of pharmaceutical & bio-industry
- by Lee, Jeong-Hwan Oct 19, 2023 05:30am
- In the audit conducted by the National Assembly Health and Welfare Committee for the Ministry of Health and Welfare and the Ministry of Food and Drug Safety this year, it was difficult to find policy questions to foster the domestic pharmaceutical and bio-industry. There are still audits from the National Health Insurance Corporation, the He
- Company
- War between the U.S. and Russia spreads Middle East risk
- by Kim, Jin-Gu Oct 19, 2023 05:29am
- Amid bloody conflicts continuing around the world, it was found that there was no direct impact on pharmaceutical export performance. The analysis is that this is because the proportion of pharmaceutical exports to countries in conflict is very small in the first place. However, it is predicted that if the conflict spreads to nearby areas due
- Policy
- HIRA ‘will discuss reimb obesity treatments with MOHW'
- by Lee, Tak-Sun Oct 19, 2023 05:29am
- In response to the claim that obesity should be recognized as a chronic disease and its treatment covered by Korea’s health insurance, President Jung-gu Kang of the Health Insurance Review and Assessment Service responded that he would discuss the request with the Ministry of Health and Welfare. Kang made this announcement at the Natio
- Company
- Korean new drug cash cows bring in KRW 100 bil in 3 quarters
- by Chon, Seung-Hyun Oct 19, 2023 05:29am
- Domestically developed drugs are continuing to show strong performance in high ranks in Korea’s outpatient prescriptions market. Prescriptions of Hanmi Pharm’s combination new drug Rosuzet and HK Inno.N's K-CAB have exceeded KRW 100 billion in just 3 quarters. Daewoong Bio's brain function enhancer Gliatamin also performed well. According
- Company
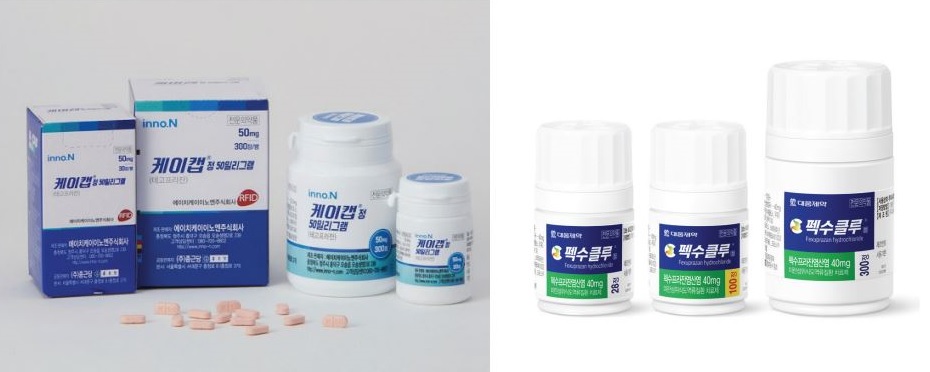
- Global 1 trillion won project
- by Kim, Jin-Gu Oct 19, 2023 05:29am
- Domestically developed new drugs in the P-CAB (Potassium-competitive gastric acid secretion inhibitor) series are accelerating their overseas expansion. HK inno.N K-CAB and Daewoong Pharmaceutical Fexuclue both set a global sales goal of 1 trillion won within five years. To this end, K-CAB has obtained product approval in 7 countries. In add
- Policy
- NA calls for prompt reimb of Ilaris during NA audit
- by Lee, Tak-Sun Oct 19, 2023 05:29am
- The National Assembly requested progress to be made in reimbursing ‘Ilaris (canakinumab, Novartis),’ a drug used to treat Hereditary recurrent fever syndromes that affect 13 patients in Korea. Rep. Sun-Woo Kang of the Democratic Party of Korea and member of the National Assembly Health and Welfare Committee suggested so at the Health I