- Opinion
- [Reporter’s View] KRPIA pulls out ‘structural reform' card
- by Eo, Yun-Ho Oct 12, 2023 05:37am
- The Korean Research-based Pharmaceutical Industry Association (KRPIA) has pulled out the ‘expenditure structure reform’ card as a solution to finance new drug expenditures. Although the message seems somewhat familiar, it is a new and unprecedented request. On the 4th, KPRIA released the results of 'A study on the analysis and rational
- Policy
- Overseas drug price comparison plan to be announced
- by Lee, Tak-Sun Oct 12, 2023 05:37am
- The final plan will likely be finalized after gathering opinions from the pharmaceutical industry. It is expected that a plan to reevaluate overseas drug prices will be revealed around the end of the year. Health authorities plan to prepare a reevaluation plan by the end of the year and then proceed with it starting next year. According to t
- Policy
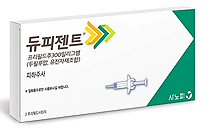
- AD drug Dupixent’s fails 1st negotiations for RSA renewal
- by Lee, Tak-Sun Oct 12, 2023 05:37am
- The first round of negotiations to renew the risk-sharing agreement (RSA) contract for the severe atopic dermatitis treatment ‘Dupixent (dupilumab, Sanofi)’ has fallen through. The company and the National Health Insurance Service plan to discuss Dupixent’s RSA renewal through additional negotiations. According to the industry on t
- Policy
- 75% of patients who administered Kymriah is not effective
- by Lee, Jeong-Hwan Oct 11, 2023 05:37am
- More than 75% of patients who received the ultra-high-priced drug Kymriah, which costs 360 million won in one dose, did not have an improvement effect. On the 6th, Kim Young-joo, a member of the National Assembly Health and Welfare Committee (the Democratic Party, Yeongdeungpo Gap) received data from the Health Insurance Review and Assess
- Policy
- ‘Little progress for NIP support of 9-valent HPV vaccines'
- by Lee, Jeong-Hwan Oct 11, 2023 05:36am
- The number of patients receiving treatment for head and neck cancer and oropharyngeal cancer, both of which are caused by HPV (human papillomavirus), is on the rise. In just a decade, from 2013 to last year, the number of head and neck cancer patients increased by 41.6%, and the number of oropharyngeal cancer patients increased by 56%. In
- Policy
- Eisai’s Jyseleca completes negotiations with NHIS for reimb
- by Lee, Tak-Sun Oct 11, 2023 05:36am
- Eisai Korea has completed negotiations with the National Health Insurance Service for its JAK inhibitor ‘Jyseleca Tab (filgotinib).’ As a result, Jyseleca is now on the verge of being listed for reimbursement in Korea. This drug is the 5th JAK inhibitor introduced to Korea. It has a mechanism of action that selectively inhibits JAK1, t
- Policy
- Hyaluronic acid eye drops, start to revise the standard
- by Lee, Tak-Sun Oct 11, 2023 05:36am
- The HIRA has started to revise the standards to limit the use of Hyaluronic acid sodium eye drops. It is known that the revision of standards will be completed as early as the end of the year, and will be implemented from next year. According to an industry example on the 10th, the HIRA has entered a review of the revision of the standards f
- Company
- Pricing negotiations for Koselugo’s reimb start after 2 yrs
- by Eo, Yun-Ho Oct 11, 2023 05:36am
- Koselugo, which is a treatment for pediatric patients with neurofibromatosis, has entered its last gateway to receiving reimbursement in Korea. According to industry sources, AstraZeneca has recently started drug pricing negotiations with the National Health Insurance Service to set the insurance price for its new neurofibromatosis drug,
- Company
- Trajenta, unregistered patent challenge spreads
- by Kim, Jin-Gu Oct 10, 2023 05:27am
- Challenges to Trajenta's unlisted patents have expanded further. The number of patents targeted by generic companies increased from 8 to 11. According to the pharmaceutical industry on the 5th, Kukje Pharmaceutical recently requested a passive rights scope confirmation trial for three Trajenta-Duo formulation patents. These three formulation
- Policy
- Tepmetko attempts reimb again in KOR
- by Lee, Tak-Sun Oct 10, 2023 05:27am
- Merck is reattempting reimbursement listing of its MET-targeted anticancer therapy ‘Tepmetko (tepotinib)’ in Korea. The drug was unable to pass the Health Insurance Review and Assessment Service’s Cancer Disease Deliberation Committee review in February. The company voluntarily withdrew its reimbursement process at the time. The c